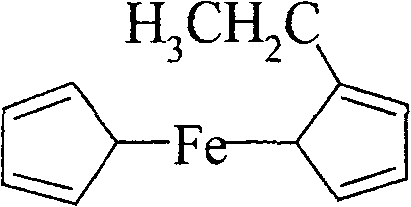Preparation method of ethyl dicyclopentadienyl iron
A technology of ethyl ferrocene and acetyl ferrocene, applied in chemical instruments and methods, metallocene, organic chemistry and other directions, can solve problems such as high cost, incomplete conversion, unsuitable for industrial production, etc., and achieves low cost , The effect of short reaction time and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 0.06mol (13.68g) acetyl ferrocene, 0.25mol (33.3g) anhydrous aluminum trichloride and 150ml chloroform to a 500ml three-necked reaction flask equipped with a thermometer, a reflux condenser and a magnetic stirring device. Stir, add 0.24mol (9.1g) sodium borohydride dropwise, heat to 50°C, react for 6 hours, cool to room temperature, add 20% ammonia water dropwise, adjust to PH=10, separate the organic phase, and use 100ml three Extracted twice with methyl chloride, combined the organic phases, washed with water until neutral, dried with anhydrous sodium sulfate, and evaporated the solvent from the dried liquid to obtain 12.3 g of crude product with a purity of 97.3% (HPLC), and distilled under reduced pressure to obtain ethyl di Ferrocene product 11.0g, yield 86.6%, purity 99.1% (HPLC), boiling point: 97-98°C / 170Pa.
Embodiment 2
[0025] Add 0.06mol (13.68g) acetyl ferrocene, 0.28mol (38.2g) anhydrous zinc chloride and 200ml 1,2-dichloro to a 500ml three-necked reaction flask equipped with a thermometer, a reflux condenser and a magnetic stirring device Ethane, stirred at room temperature, added dropwise 0.26mol (14g) potassium borohydride, heated to 60°C, reacted for 3.5 hours, cooled to room temperature, added dropwise 20% ammonia water, adjusted to PH = 12, separated the organic phase and the aqueous phase Extract twice with 100ml of 1,2-dichloroethane, combine the organic phases, wash with water until neutral, dry with anhydrous sodium sulfate, and evaporate the solvent from the dried solution to obtain 12.5 g of crude product with a purity of 97.8% (HPLC). Distilled under reduced pressure to obtain 11.6 g of ethyl ferrocene product, with a yield of 90%, a purity of 99% (HPLC), and a boiling point of 97-98°C / 170Pa.
Embodiment 3
[0027] Add 0.06mol (13.68g) acetyl ferrocene, 0.21mol (28.6g) anhydrous zinc chloride and 180ml 1,2-dichloro to a 500ml three-necked reaction flask equipped with a thermometer, a reflux condenser and a magnetic stirring device Ethane, stirred at room temperature, added dropwise 0.2mol (7.6g) sodium borohydride, heated to 65°C, reacted for 4.5 hours, cooled to room temperature, added dropwise 20% ammonia water, adjusted to PH = 11, separated the organic phase, water The phase was extracted twice with 100ml of 1,2-dichloroethane, the organic phases were combined, washed with water until neutral, dried with anhydrous sodium sulfate, and the dry solution was evaporated to remove the solvent to obtain 12.3g of crude product with a purity of 98.1% (HPLC) , Distilled under reduced pressure to obtain 11.8g of ethyl ferrocene product, yield 92%, purity 99.2% (HPLC), boiling point: 97~98°C / 170Pa.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com