Functional method for generating or screening for ligands which modulate steroid hormone receptors
A functional, receptor technology that can be used in compound screening, chemical instruments and methods, biological testing, etc., and can solve problems such as low overall homology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0196] Example 1: Combination of MNAR and VDR
[0197] method
[0198] Reagent
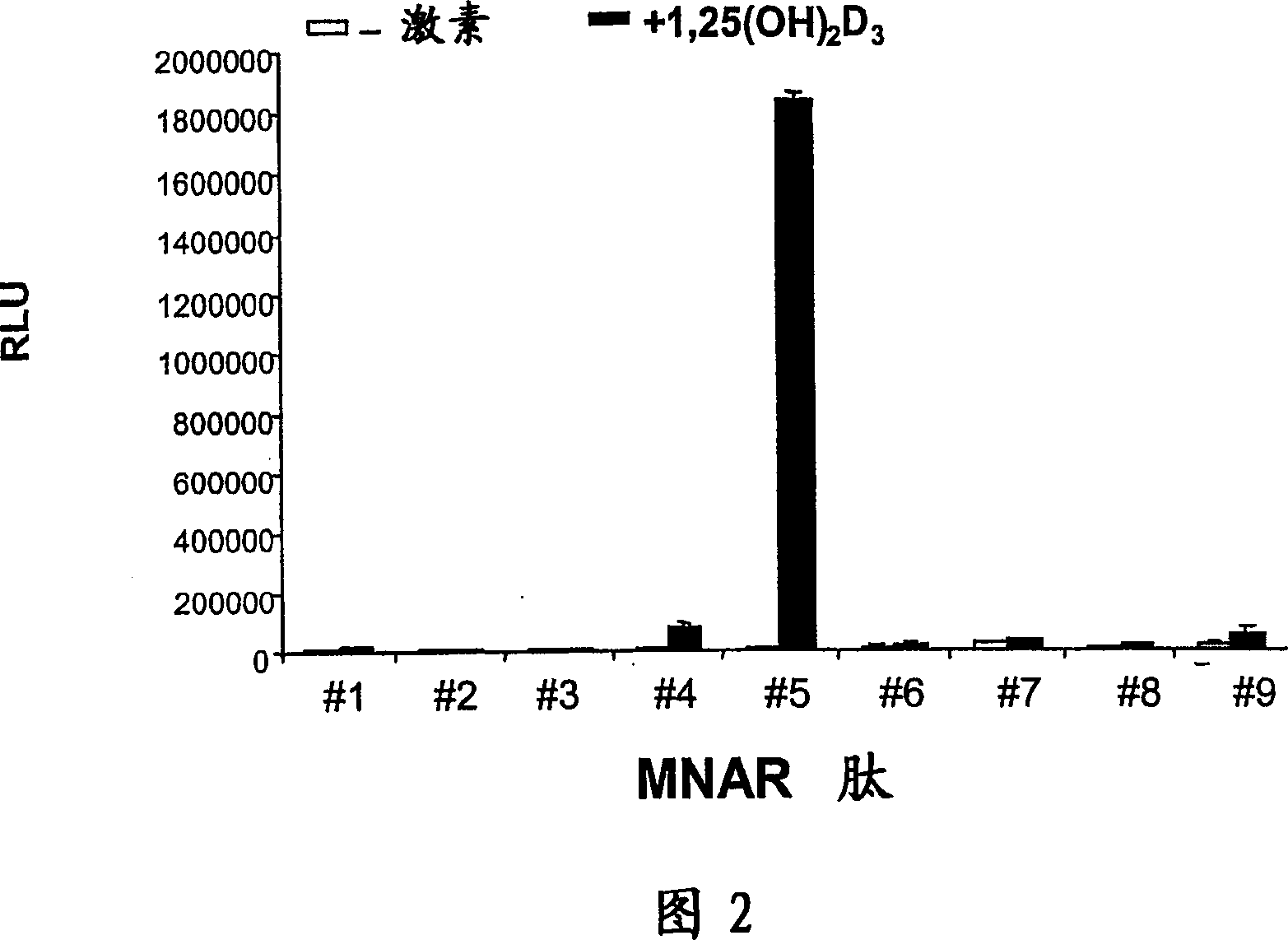
[0199] 1,25(OH) 2 Vit D3 was purchased from Sigma. Biotinylated peptides corresponding to different MNAR LXXLL motifs were synthesized and purified by peptide chemistry at Wyeth Research. Glutathione Sepharose beads were obtained from Sigma. Anti-Phosphotyrosine Antibody, SuperSignal Elisa Pico Peroxidase Substrate and Reacti-Bind TM Neutr Avidin TM Coated microtiter plates were from Pierce.
[0200] GST pull dow interaction analysis
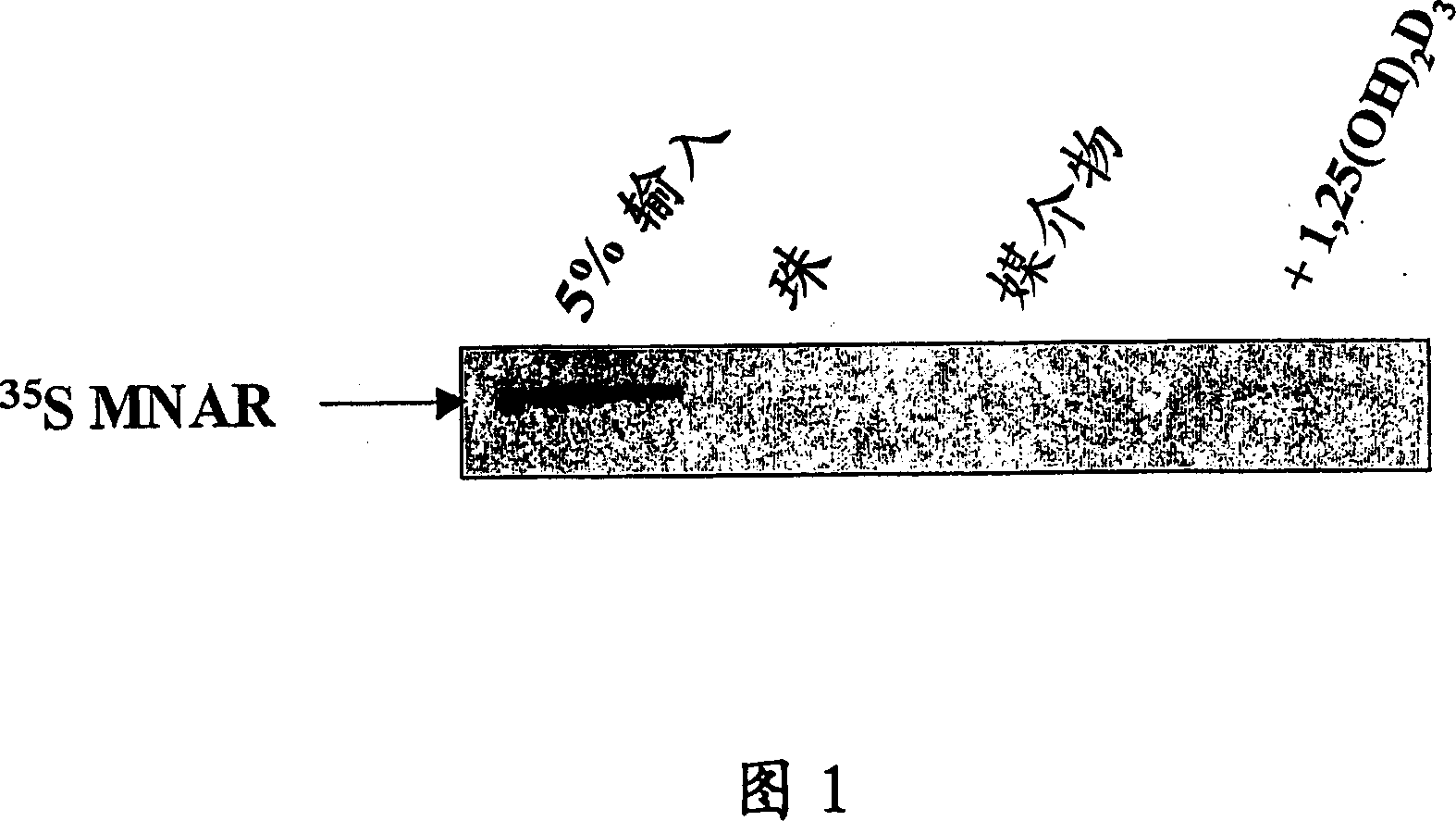
[0201] A GST fusion of the VDR ligand-binding domain (i.e., amino acids 110-427) (designated GST-VDR-LBD) was expressed in BL-21 cells and bound to Glutathione Sepharose-4B (Amersham Biosciences, Piscataway, NJ). .Wild-type MNAR was transcribed / translated with the Promega TNT Quick CoupledTranscription / Translation System (Madison, WI) and performed 35 S radiolabeled, then in 100 nM 1,25(OH) in binding buffer (50 mM Tris pH 8.0, 150 mM NaCl, 1 mM DTT, 1 mM P...
Embodiment 2
[0209] Example 2: VDR activity is activated by agonists of MNAR
[0210] method
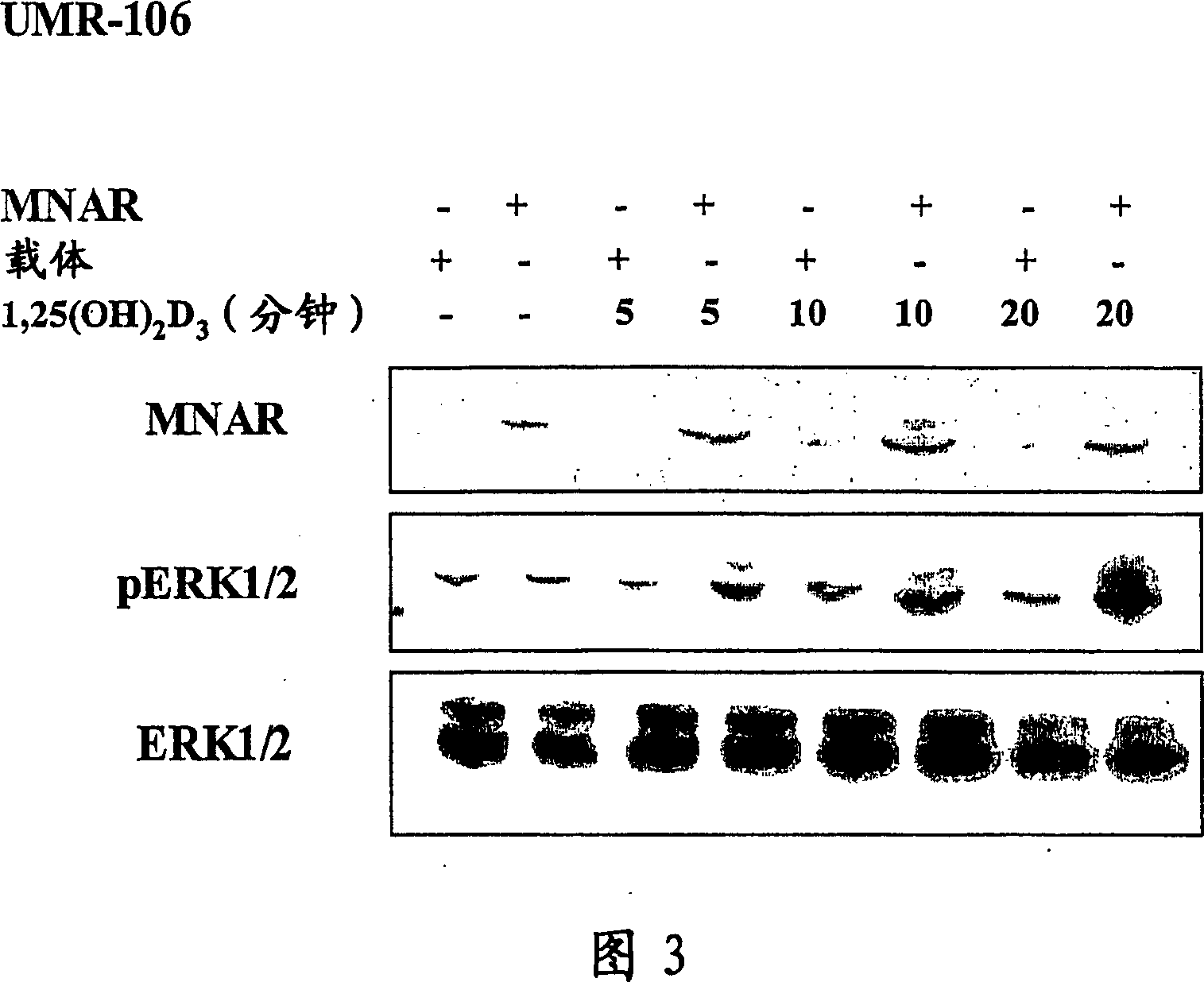
[0211] Western blot analysis
[0212] UMR-106 cells were transfected with control vector or MNAR expression vector using Lipofectamine 2000 reagent according to the manufacturer's recommended method. After transfection, cells were cultured for an additional 48 h in medium supplemented with 2% charcoal-adsorbed FBS. After 48 hours, cells were treated with 10 nM 1,25(OH)2D3 for the indicated times. Cells were rinsed and harvested in cold PBS, then centrifuged and the supernatant discarded. Use 2 volumes of lysis buffer (20mM Tris-HCl pH7.5, 150mM NaCl, 1mMNa2EDTA, 1mM EGTA, 1% Triton, 2.5mM sodium pyrophosphate, 1mM β-glycerophosphate, 1mM Na 2 VO 4 , 1 μg / μl leupeptin) to lyse cells. Cell debris was removed by centrifugation and equal amounts of protein were subjected to SDS-PAGE. Separated proteins were transferred to nitrocellulose membranes and the levels of MNAR, ERK and p-ERK were dete...
Embodiment 3
[0221] Example 3: Regulation of PI3 Kinase in ER by MNAR
[0222] method
[0223] MNAR was recently shown to affect PI3 / Akt kinase-mediated signaling in the ER by forming an ER / MNAR / PI3 kinase ternary complex. MNARs are expected to have similar effects on PI3 / Akt kinases that signal through the VDR.
[0224] Transfection and cell lysate preparation
[0225] MCF-7 cells were transfected with control, MNAR expression vector, non-specific siRNA or MNAR-specific siRNA using Lipofectamine 2000 reagent following the manufacturer's recommended method. After transfection, cells were cultured for an additional 48 hours in medium supplemented with 2% charcoal-adsorbed FBS. After 48 hours, cells were treated with 10 nM 17[beta]-estradiol for the indicated times. Cells were rinsed and harvested in cold PBS, then centrifuged and the supernatant discarded. Use 2 volumes of lysis buffer (20mM Tris-HCl pH7.5, 150mM NaCl, 1mM Na 2 EDTA, 1mM EGTA, 1% Triton, 2.5mM sodium pyrophosphate, 1m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com