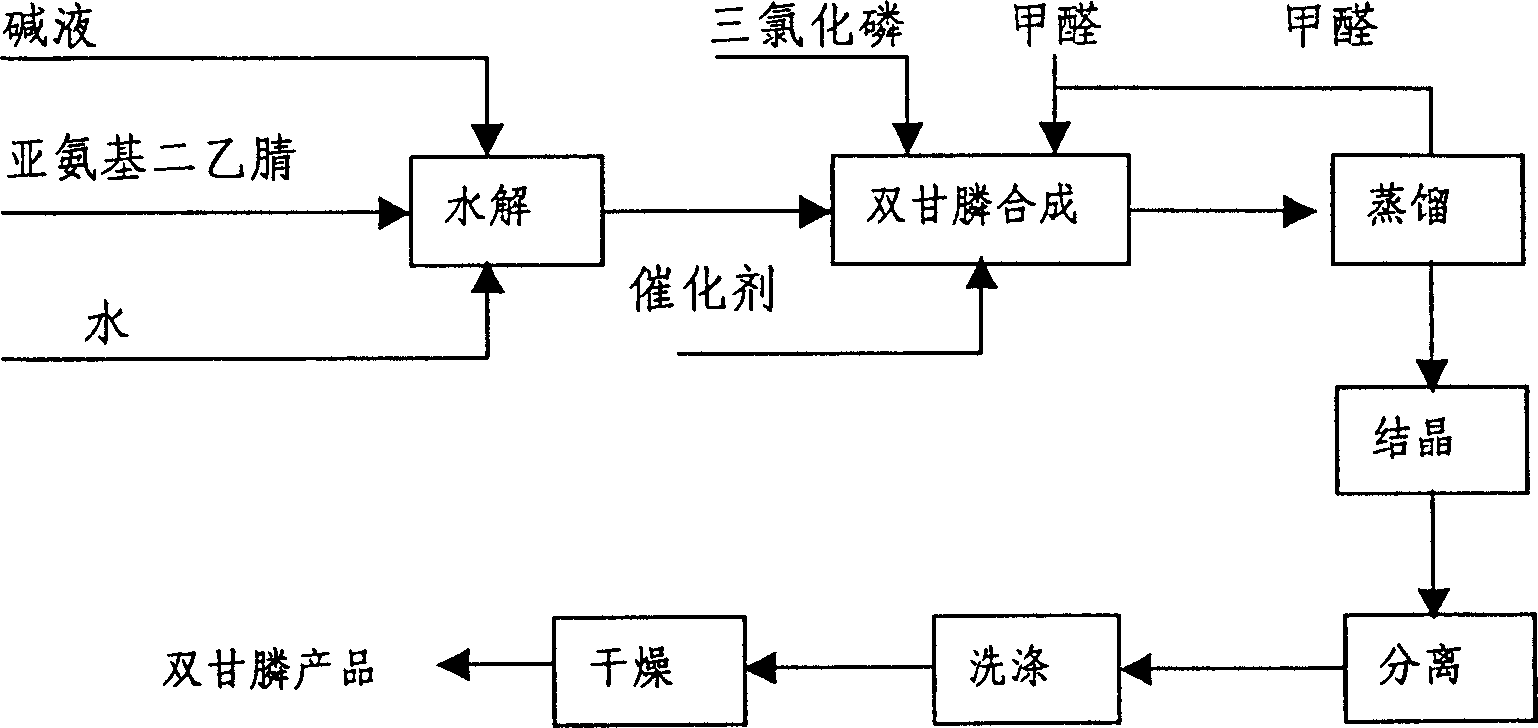
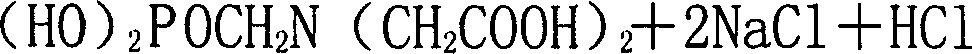Method for preparing Phosphonomethyl iminodiacetic acid (PMIDA) through hydrolysis of imino diacetonitrile
A technology of iminodiacetonitrile and iminodiacetate, which is applied in the field of preparation of bisglyphosate by hydrolysis of iminodiacetonitrile, can solve problems such as difficult recycling, increased waste water volume, lengthened process, etc., and achieves low manufacturing cost and equipment saving Investment and energy consumption, the effect of short process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 84 grams of sodium hydroxide and 340 grams of water are mixed into a 20% sodium hydroxide solution (potassium hydroxide solution or other lye can also be used) with a mass percentage composition. Raise the temperature to 80°C, add 120 grams of iminodiacetonitrile with a mass percentage of 95% in four batches under the conditions of stirring and pressure of -0.01 to -0.04 MPa, and keep warm for 2 hours to obtain iminodiacetic acid Disodium salt solution, the released ammonia is absorbed by water. Add 50 grams of hydrochloric acid with a mass percentage of 36%, maintain the temperature at 95 to 105°C, control the pressure at -0.01 to -0.04MPa, add 168 grams of phosphorus trichloride with a mass percentage of 98%, and release HCl gas is absorbed by water. Then be warming up to 103 ℃, add 50 gram mass percentage composition and be 36% hydrochloric acid, dropwise add 162 gram mass percentage composition be 37% formaldehyde, after formaldehyde is added, maintain temperature ...
Embodiment 2
[0038] 85 grams of sodium hydroxide was mixed with 340 grams of water to make a 20% sodium hydroxide solution. Raise the temperature to 80° C., under the conditions of stirring and pressure of -0.01 to -0.04 MPa, add a total of 120 grams of 95% iminodiacetonitrile in four batches, and keep warm for 2 hours to prepare a solution of iminodiacetic acid disodium salt. The released ammonia is absorbed by water. Add 50 grams of 36% hydrochloric acid, control the pressure at -0.01 to -0.04 MPa, and add 168 grams of 98% phosphorus trichloride dropwise at a temperature of 50 to 80° C., and the released HCl gas is absorbed by water. Then the temperature was raised to 105° C., and 146 grams of 37% formaldehyde was added dropwise. After the formaldehyde was added, the temperature was maintained at 110° C. for 2 hours to complete the synthesis reaction of diglyphosate. After the synthesis liquid was processed, 201.4 grams of bisglyphosate with a purity greater than 98% were obtained. Oth...
Embodiment 3
[0040] 84 grams of sodium hydroxide was mixed with 340 grams of water to make a 20% sodium hydroxide solution. Raise the temperature to 80° C., under the conditions of stirring and pressure of -0.01 to -0.04 MPa, add a total of 120 grams of 95% iminodiacetonitrile in four batches, and keep warm for 2 hours to prepare a solution of iminodiacetic acid disodium salt. The released ammonia is absorbed by water. Under the maintenance temperature of 50-80°C, the pressure is controlled at -0.01--0.04 MPa, and 173 grams of 98% phosphorus trichloride is added dropwise, and the released HCl gas is absorbed by water. Then the temperature was raised to 110° C., and 146 grams of 37% formaldehyde was added dropwise. After the formaldehyde was added, the temperature was maintained at 110° C. for 2 hours to complete the synthesis reaction of diglyphosate. The synthetic solution was treated to obtain 207.7 grams of diglyphosate. Other operating conditions are with embodiment 1. The yield of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com