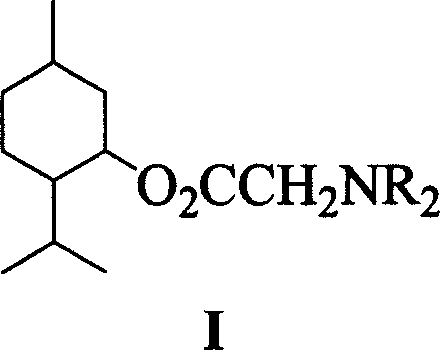
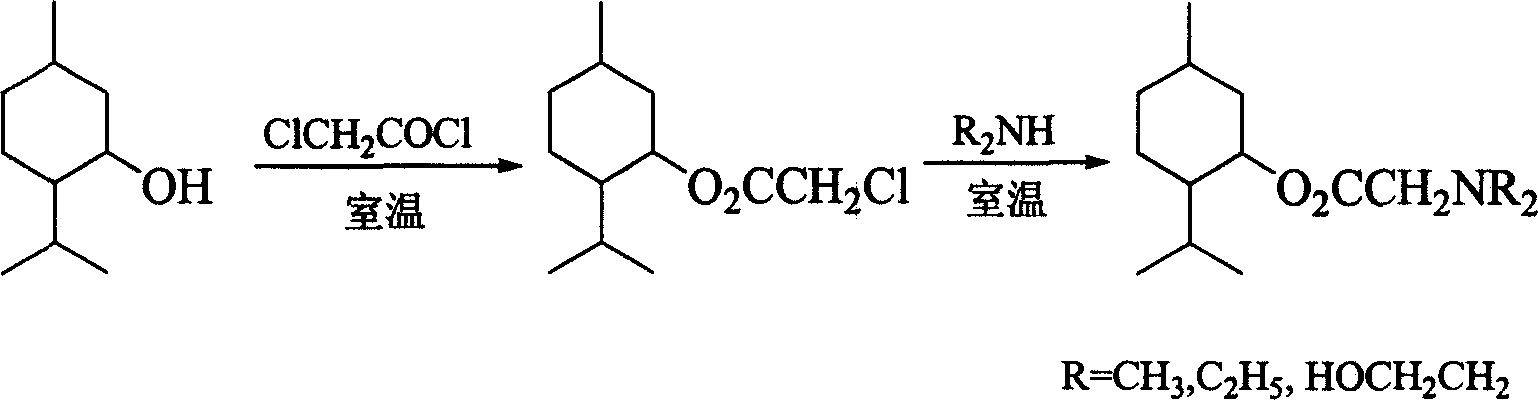Derivative of new type menthol, preparation method and application
A menthol derivative and menthol technology are applied in the direction of cyanide reaction preparation, organic compound preparation, chemical instruments and methods, etc., which can solve the problems of cumbersome operation and increased cost of synthesis reaction, so as to enhance drug efficacy and promote economic development. The effect of skin absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Invention of the compound and its synthesis, taking N,N-dimethylglycine menthyl ester as an example.
[0035] (1) Synthesis of Menthyl Chloroacetate
[0036] In a 50 mL three-neck flask, place 4.69 g of menthol, and add 2.6 mL of chloroacetyl chloride dropwise while stirring at room temperature, and continue stirring at room temperature for 2 h after the dripping is completed. The reaction solution was saturated NaHCO 3 The solution was adjusted to weak alkalinity, the organic layer was separated, the aqueous layer was extracted with ether or ethyl acetate, the combined organic layers were washed with saturated brine, and anhydrous Na 2 SO 4 Dry overnight, evaporate the solvent, and perform column chromatography (silica gel: 100-300 mesh), using chloroform as the eluent, and evaporate the eluent to obtain 5.81 g of a colorless liquid with a yield of 83.2%. The infrared spectrum data of product are as follows:
[0037] IR (KBr) v: 2957, 2871, 1758, 1736...
Embodiment 2
[0041] Embodiment 2: Invention of the compound and its synthesis, taking N,N-diethylglycine menthyl ester as an example.
[0042] (1) Synthesis of Menthyl Chloroacetate
[0043] In a 50mL three-necked flask, add 1.56g menthol, 1.04g chloroacetic acid and 25mL cyclohexane respectively, and add catalyst 0.15g SnCl 2 Solid, reflux 2h, filter, and the filtrate is saturated with Na 2 CO 3 The solution was adjusted to be weakly alkaline, the organic layer was separated, the aqueous layer was extracted with ether, the combined organic layers were washed with saturated brine, and anhydrous Na 2 SO 4 Dry overnight, evaporate the solvent, use chloroform as the eluent, perform column chromatography (silica gel: 100-300 mesh), evaporate the eluent, and obtain 2.06 g of a colorless liquid with a yield of 88.9%.
[0044] (2) Synthesis of N, N-diethylglycine menthyl ester
[0045] Add 7.8 mL of diethylamine to 6.98 g of menthol chloroacetate, stir the reaction at room temperature for 2 ...
Embodiment 3
[0047] Embodiment 3: Invention of the compound and its synthesis, taking N,N-dimethylglycine menthyl ester as an example.
[0048] In a 50mL three-necked flask, add 6.98g menthol, 3.71g N,N-dimethylglycine and 25mL cyclohexane respectively, and add 0.51g SnCl 2 Solid, refluxed for 2.5h, filtered, and the filtrate was washed with saturated Na 2 CO 3 The solution was adjusted to be weakly alkaline, the organic layer was separated, the aqueous layer was extracted with ether, the combined organic layers were washed with saturated brine, and anhydrous Na 2 SO 4 Dry overnight, evaporate the solvent, use chloroform as the eluent, perform column chromatography (silica gel: 100-300 mesh), evaporate the eluent, and obtain 9.37 g of a colorless liquid with a yield of 86.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com