Monomer, and method for using it to fabricate LCD faceplate
A monomer and substrate technology, applied in liquid crystal materials, chemical instruments and methods, optics, etc., can solve problems such as lattice defects, affecting the display quality of liquid crystal display panels, and different diffusion distances between reactive monomers and liquid crystal molecules.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
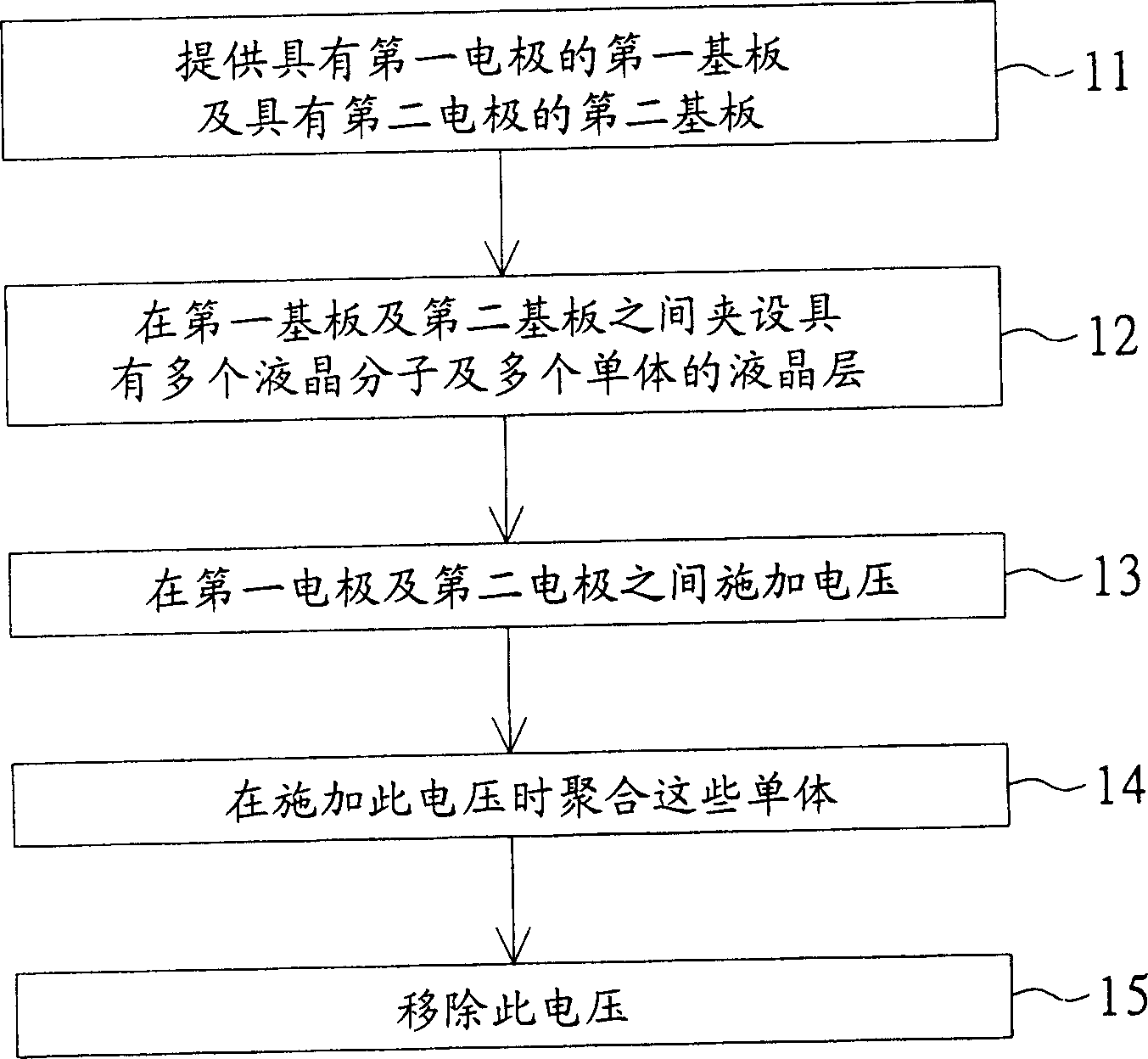
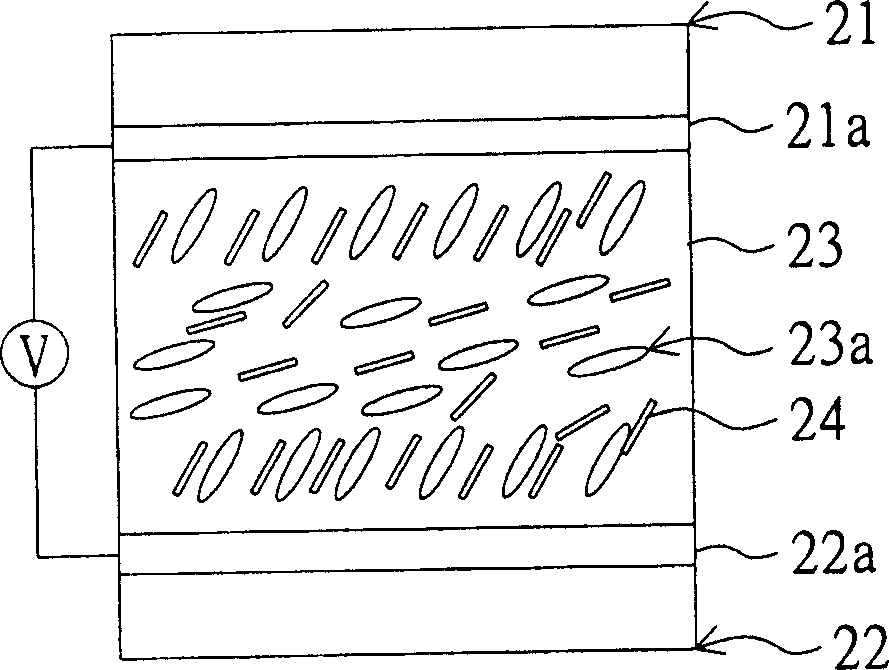
Examples
no. 1 example
[0063] The first synthesis example: a kind of embodiment in chemical formula [11]
[0064] (1-1) Synthesis of 4-hydroxy-4'-ethoxybiphenyl (4-hydroxy-4'-ethyloxybiphenyl), as shown in chemical formula [51]:
[0065]
[0066]First, the reaction bottle was filled with argon gas, and 8 mmol (mmol), 60% by weight sodium hydride and 200 ml of dry dimethoxyethane (dimethoxyethane, DME) were put into the reaction bottle. Next, when the reaction solution was cooled by ice cubes, 2 mmol of 4,4'-dihydroxybiphenyl (4,4'-dihydroxybiphenyl) was added to the reaction solution. Then, the reaction solution was heated to room temperature, and the reaction solution was continuously stirred for 2 hours. Next, the reaction solution was cooled to minus 60°C. Then, 1 mmol of bromoethane was gradually dropped into the reaction solution. Then, the reaction solution was heated to room temperature, and the reaction solution was continuously stirred until the next day. Then, the reaction solution ...
no. 2 example
[0070] The second synthesis example: a kind of embodiment in the chemical formula [17]
[0071] (2-1) Synthesis of 4-(2-hydroxyethoxy)-4'-ethoxybiphenyl (4-(2-hydroxyethyloxy)-4'-ethyloxybiphenyl), as shown in the chemical formula [53]:
[0072]
[0073] First, the reaction flask was filled with argon gas, and 4 mmol, 60% by weight of sodium hydride and 200 ml of dry dimethoxyethane were put into the reaction flask. Next, when the reaction solution was cooled by ice cubes, 1 mmol of 4-hydroxy-4'-ethyloxybiphenyl (4-hydroxy-4'-ethyloxybiphenyl) was added to the reaction solution. Then, the reaction solution was heated to room temperature, and the reaction solution was continuously stirred for 2 hours. Next, the reaction solution was cooled to minus 60°C. Then, 1.5 mmol of bromoethanol was gradually dropped into the reaction solution. Then, the reaction solution was heated to room temperature, and the reaction solution was continuously stirred until the next day. Then, th...
no. 3 example
[0077] The third synthesis example: a kind of embodiment in chemical formula [23]
[0078] (3-1) has the same steps as (2-1) to obtain 4-(2-hydroxyethoxy)-4'-ethoxybiphenyl, which will be omitted here.
[0079] (3-2) Synthetic monomer, as shown in chemical formula [55]:
[0080]
[0081] First, the reaction flask was filled with argon gas, and 4 mmol, 60% by weight of sodium hydride and 200 ml of dry dimethoxyethane were put into the reaction flask. Then, when the reaction solution was cooled by ice cubes, 1 mmol of 4-(2-hydroxyethoxy)-4'-ethoxybiphenyl was added to the reaction solution. Then, the reaction solution was heated to room temperature, and the reaction solution was continuously stirred for 2 hours. Next, the reaction solution was cooled to minus 60°C. Then, 1.5 mmol of 3-bromo-2-fluoroprop-1-ene (3-bromo-2-fluoroprop-1-ene) was gradually dropped into the reaction solution. Then, the reaction solution was heated to room temperature, and the reaction solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com