Production of gabapendin
A technology of gabapentin and gabapentin salt, which is applied in the field of preparation of gabapentin, can solve the problems of high equipment requirements, high energy consumption, difficult content control, and complicated process, and achieve the effects of easy operation, low energy consumption, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
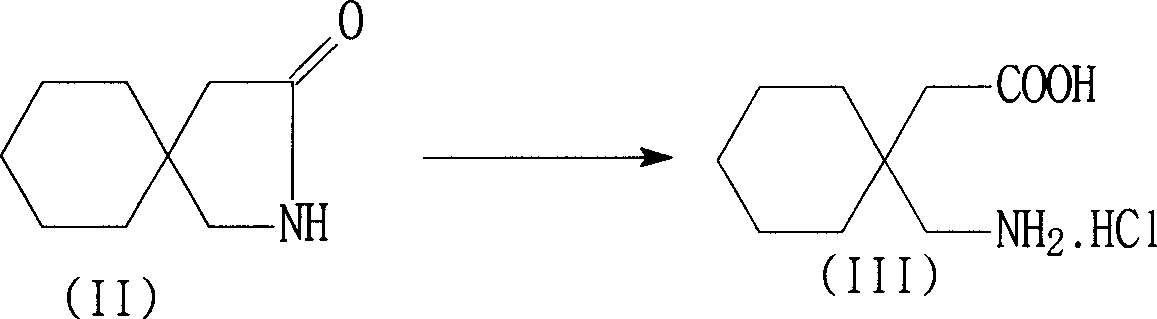
[0042] Embodiment 1 Preparation of gabapentin hydrochloride
[0043] In a 2000ml reaction flask, add 167g of 3,3-pentamethylenebutyrolactam, 900g of 31% concentrated hydrochloric acid and 200ml of purified water, stir, heat up and reflux for 5 hours, and cool the reaction solution to -5~5 ℃, filtered to obtain the wet product of white crystal gabapentin hydrochloride, which is equivalent to 206.5 grams of dry product (water of crystallization is measured and deducted by the Karl-Fisher method), yield 91.1%, purity 98.5%, 3,3-pentamethylene butyl Lactam content 0.8%. The concentration of the mother liquor hydrochloric acid aqueous solution is about 15% to 18%. A part of the mother liquor (A) is supplemented with 36% concentrated hydrochloric acid for use in the next batch, and the remaining mother liquor (B) is used to recover 3,3-pentylidenebutyrolactam.
Embodiment 2
[0044] Embodiment 2 Preparation of gabapentin hydrochloride
[0045] In a 2000 ml reaction flask, add 167 grams of 3,3-pentylidene butyrolactam, 900 grams of the mother liquor (A) containing hydrochloric acid in Example 1, and add 300 grams of 36% concentrated hydrochloric acid. Stirring, heating and reflux reaction for 5 hours, the reaction solution was cooled to -5~5°C, filtered to obtain the wet product of white crystal gabapentin hydrochloride, which was equivalent to 216 grams of dry product (the water of crystallization was measured and deducted by the Karl-Fisher method), and the The yield is 95.3%, the purity is 98.6%, and the impurity 3,3-pentylidenebutyrolactam content is 0.75%.
Embodiment 3
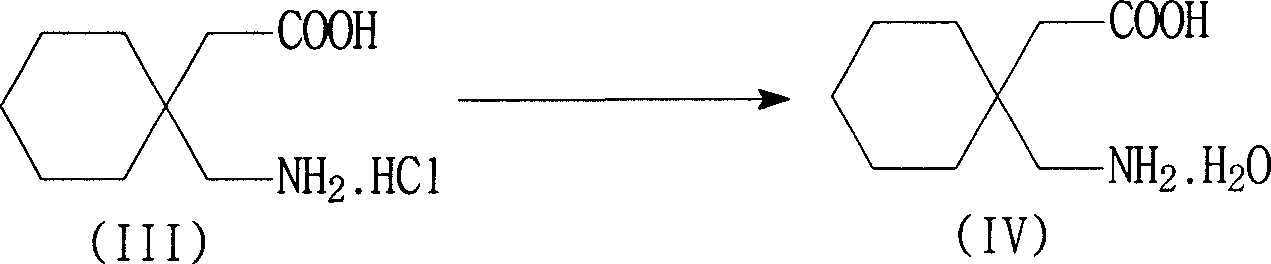
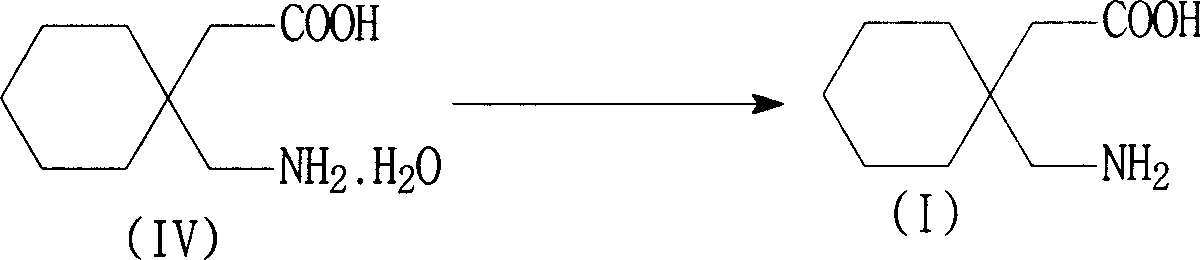
[0046] Example 3 Preparation of Gabapentin Hydrate
[0047] In a 2000 ml reaction bottle, add 300 ml of purified water, the wet product gabapentin hydrochloride (equivalent to 206 grams of dry product) of Example 1, stir evenly, add 30% sodium hydroxide dropwise at 15~25 ° C, adjust pH =4~6, dissolve, add 1.5g of activated carbon and 0.5g of diatomaceous earth, stir for 0.5 hours, filter, adjust the pH of the filtrate to 8.0~8.5 with 30% sodium hydroxide aqueous solution at 0~10°C, white crystals precipitate, continue to cool to 0-5°C, filter, and wash with a small amount of ice water to obtain the wet product of gabapentin hydrate, which is equivalent to 160 grams of dry product, with a yield of 85.2%, a purity of 99.5%, and an impurity 3,3-pentylidene butyrolactam content <0.1 %. The separated mother liquor (C) is used to recover 3,3-pentylidenebutyrolactam.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com