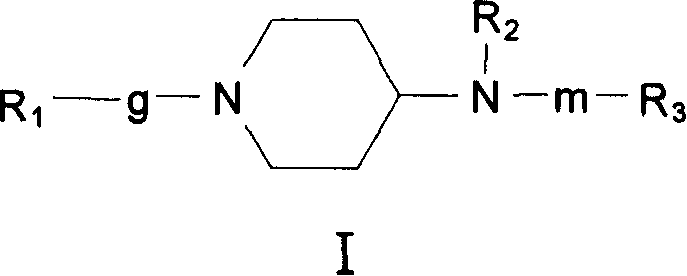
4-amino piperidine compounds and their pharmaceutical use
A technology of aminopiperidine and compounds, applied in the field of 4-aminopiperidine compounds, which can solve problems such as addiction and poor pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] Example 1: N-(3-methyl-2-butene-1-yl)-N-benzyl-1-(4-dimethylaminobenzyl)-piperidin-4-amine trihydrochloride synthesis
[0169] 1.1 Dissolve 1.07g (10mmol) benzylamine in 25ml dichloromethane, and add 1.99g (10mmol) 1-tert-butoxycarbonyl-4-piperidone under stirring. Stir at room temperature for 30 min. Cool to 0°C, add 3.18g (15mmol) NaBH(OAc) in portions 3 After the addition, the reaction solution was raised to room temperature, and the stirring reaction was continued for 18h. Spot the plate to monitor the reaction, developer: ethyl acetate-petroleum ether=1:1. Add 25ml of dichloromethane, wash with 2×50ml of saturated sodium bicarbonate solution, and once with 50ml of saturated sodium chloride solution. An appropriate amount of anhydrous sodium sulfate was added to the dichloromethane layer to dry overnight. After filtering, the solvent was distilled off to obtain a yellow-brown oily viscous substance. Silica gel column separation, eluent: dichloromethane-methano...
Embodiment 2
[0173] Example 2: N-(3-methyl-1-butyl)-N-(4-benzyloxybenzyl)-1-(4-dimethylaminobenzyl)-piperidin-4-amine trisalt salt synthesis
[0174] 2.1 Dissolve 1.99g (10mmol) of 1-tert-butoxycarbonyl-4-piperidone in 40ml of dichloromethane, add 0.87g (10mmol) of 3-methyl-butylamine (R 2 NH 2 ), stirred at room temperature for 2h. Add 3.18g (15mmol) NaBH(OAc) in portions under ice-cooling 3 After the addition, the reaction solution was raised to room temperature, and the stirring reaction was continued for 18h. Spot the plate to monitor the reaction, developer: dichloromethane-methanol = 15:1. Add 40ml of dichloromethane, wash with saturated sodium bicarbonate solution 80ml×2, saturated sodium chloride solution 80ml×1 time. An appropriate amount of anhydrous sodium sulfate was added to the dichloromethane layer to dry overnight. After filtering, the solvent was distilled off to obtain a yellow-brown oily viscous substance. Silica gel column separation, eluent: dichloromethane-meth...
Embodiment 3
[0178] Example 3: Synthesis of N-(3-methyl-2-buten-1-yl)-1-(4-dimethylaminobenzyl)-piperidin-4-amine trihydrochloride
[0179] 3.1 Add 27.2g (0.147mol) phthalimide potassium salt and 270ml DMF into a 250ml two-necked flask, and add 18.5g (0.124mol) 4-bromo-2-methyl-2- butene. Keep the internal temperature at 120°C and stir the reaction for 4h. After cooling to room temperature, 400ml of water and 400ml of dichloromethane were added. The aqueous phase was extracted with 400ml of dichloromethane x 2 times, the extract was washed with 250ml of 0.2mol / L NaOH aqueous solution x 4 times, and washed with 400ml of saturated sodium chloride x 1 time. The dichloromethane layer was separated, and an appropriate amount of anhydrous sodium sulfate was added to dry it overnight. Filtrate, evaporate the solvent under reduced pressure, precipitate a solid, and recrystallize with petroleum ether at 60-90°C to obtain 22.2 g of white crystals, which are N-(3-methyl-2-ene-butyl)-phthaloyl Ami...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com