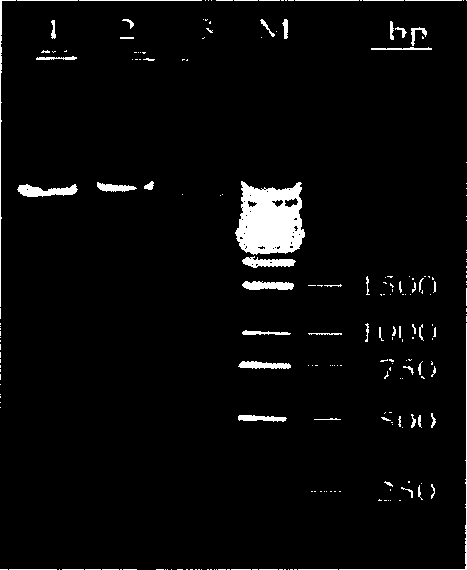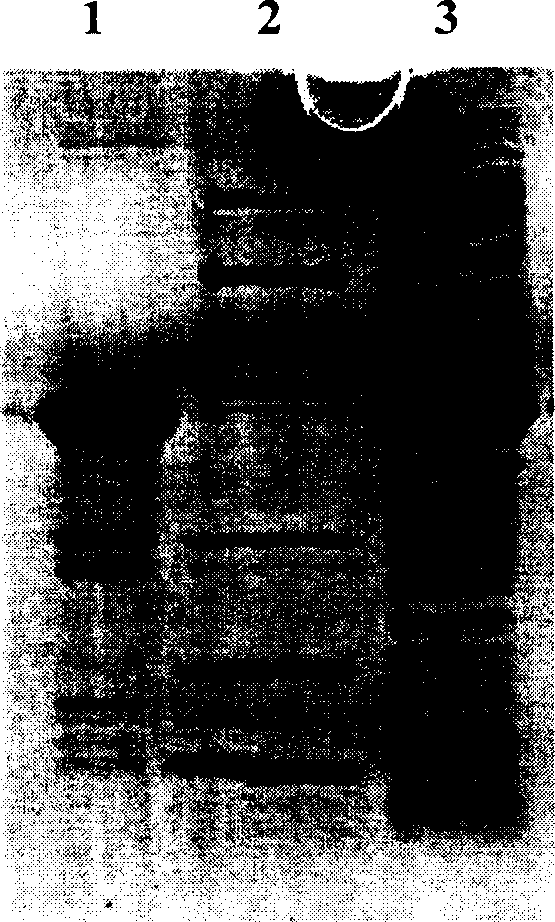Toxophasma gondii detecting kit based on recombined antigen
A technology of Toxoplasma gondii and a kit, which is applied in the field of Toxoplasma gondii detection kits, can solve the problems of high toxicity of host bacteria, lack of modification function, no specific immunoreactivity, etc., and achieves the effect of good immune activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1 Construction of pET32a(+)-tSAG1
[0075] Extract the recombinant plasmid DNA of the transformed bacteria, perform 1% agarose electrophoresis with the empty plasmid DNA, and then carry out single- and double-enzyme digestion for identification. The pET32a(+)-tSAG1 positive recombinant plasmid can be digested with Nco I and the size is about 6600bp The left and right single bands can produce two bands of about 5900bp and 700bp after double digestion with Nco I+HindIII, which is consistent with the expected results. However, pET32a (+) has only a band of 5900bp after Nco I single enzyme digestion, the above results show that the pET32a (+)-tSAG1 recombinant plasmid ( figure 1 ).
Embodiment 2
[0076] Example 2 Induced expression of engineering bacteria pET32a(+)-tSAG1 / BL21
[0077] After pET32a(+)-tSAG1 / BL21 was induced by IPTG, a specific expression band appeared at about 40kDa by SDS-PAGE analysis, which basically coincided with the expected molecular weight. The empty vector expressed thioredoxin (Thioredoxin, Trx) at the position of 21kDa, while the empty vector and the recombinant plasmid had no specific band of expressed protein before induction. After the recombinant bacteria were broken by ultrasonic waves, they were centrifuged at 14,000rpm for 20min, and the supernatant and precipitate were collected for SDS-PAGE. The results showed that the target protein was mainly expressed in soluble form. The target protein accounted for 34.36% of bacterial protein. ( figure 2 )
Embodiment 3
[0078] Purity of rSAG1 after Ni column purification of embodiment three
[0079] A large amount of recombinant protein is expressed under optimized conditions. After ultrasonic destruction, most of the recombinant protein exists in the ultrasonic supernatant in a soluble form. After the recombinant protein in the supernatant is purified by Ni-NTA, the purity can reach more than 60% %, the purified recombinant protein can obtain a purity of more than 90% after being purified by Sephadex-G75 (Fig. 3).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com