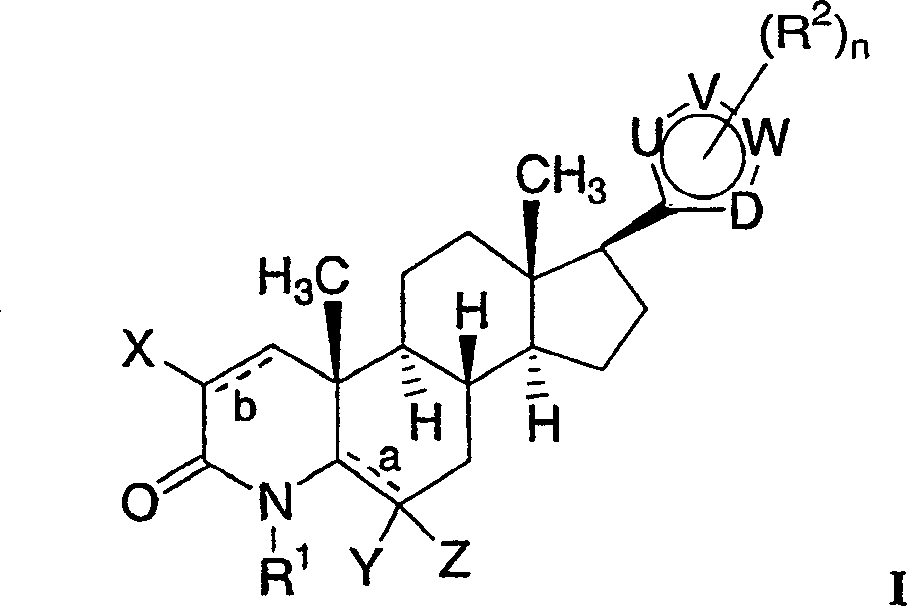
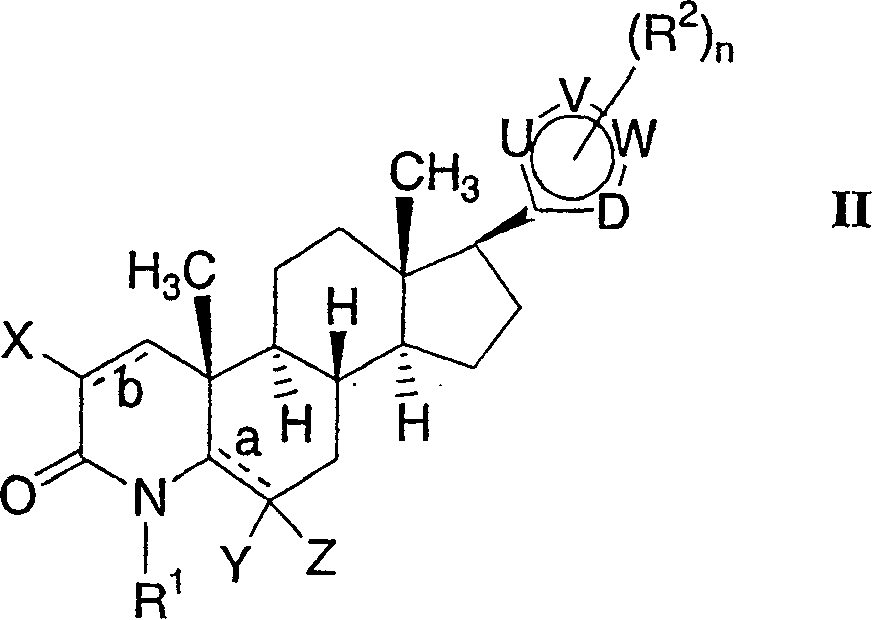
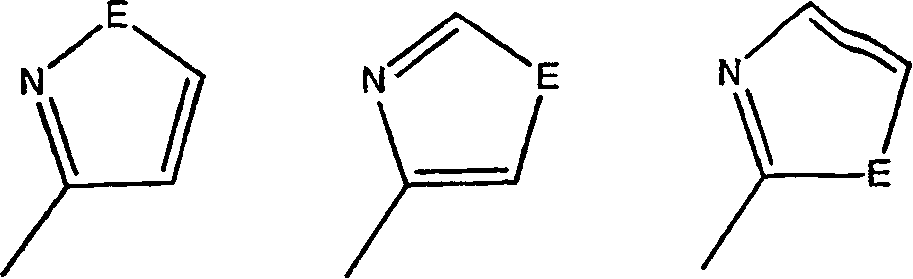
17-heterocyclic-4-azasteroid derivatives as androgen receptor modulators
一种杂环基、杂环烷基的技术,应用在17-杂环-4-氮杂甾类衍生物领域,能够解决降低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0933] Step A: N,O-(Dimethylhydroxy)-4-methyl-3-oxo-4-aza-5α-androst-1-ene- 17β-formamide (1-2)
[0934] at 0°C to 1-1 (36.5g, 110.12mmol) in CH 2 Cl 2 (500mL) solution was added Et 3 N (74.0 mL, 528.6 mmol). Isobutyl chloroformate (21.4 mL, 165.2 mmol) was added dropwise, after 15 minutes the cooling bath was removed and the reaction was stirred for 45 minutes. The reaction was then cooled to 0 °C and Et was added 3 N (55.2 mL, 396.4 mmol) and N,O-dimethylhydroxylamine hydrochloride (32.2 g, 330.4 mmol). The reaction was warmed to room temperature and stirred for 12 hours. The reaction was quenched by dropwise addition of 1N NaOH solution (100 mL) and washed with CH 2 Cl 2 (900 mL) extraction. The organic layer was washed with brine, dried (MgSO 4 ) and then concentrated. Dissolve the residue in a minimum amount of CH 2 Cl 2 in combination with Et 2 O grinding. The solid was collected and washed with Et 2 O washed and dried under high vacuum to give a whit...
Embodiment 32
[0956] Step A: 17β-[2-(1-Methyl-1H-imidazole-5-amido)-1,3-thiazol-4-yl]-4-methanol Base-4-aza-5α-androst-1-en-3-one (2-2)
[0957] to room temperature 1-30 (0.100g, 0.26mmol) in 1:1 pyridine:CH 2 Cl 2 (5 mL) was added 1-methyl-1H-imidazole-5-carbonylchloride (0.075 g, 0.52 mmol) and DMAP (0.005 g, 0.03 mmol) and the reaction was stirred for 14 hours. The reaction was concentrated and the residue was purified by silica gel chromatography (0-100% EtOAc in hexanes) to give a white solid 2-2 .
[0958] MS calcd M+H: 494, found 494.
[0959] Examples 33-50 in Table 2 were prepared in a manner similar to Example 32, except that the appropriate acid chloride, alkyl chloroformate, or sulfonyl chloride was used to generate the amides, carbamates, sulfamates as described.
[0960] Table 2
[0961]
[0962]
[0963]
[0964]
[0965]
[0966] Flowchart 3
[0967]
Embodiment 51
[0969] Step A: 17β-[2-(ureido)-1,3-thiazol-4-yl]-4-methyl-4-aza-5α-androst- 1-en-3-one (3-2)
[0970] to room temperature 1-30 (2.100g, 5.5mmol) in CH 2 Cl 2 (50 mL) was added N,N-carbonyldiimidazole (1.06 g, 6.5 mmol) and the reaction was stirred for 3 hours. Ammonia (13.6 mL, 27.2 mmol, 2M in MeOH) was added dropwise and the reaction was stirred for 18 hours. The reaction was concentrated and the residue was purified by silica gel chromatography (0-100% EtOAc in hexanes) to give a white solid 3-2 .
[0971] MS calcd M+H: 429, found 429.
[0972] Examples 52-60 in Table 3 were prepared in a similar manner to Example 51 except using the appropriate amine to yield the desired urea.
[0973] table 3
[0974]
[0975]
[0976]
[0977]
[0978] Flowchart 4
[0979]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com