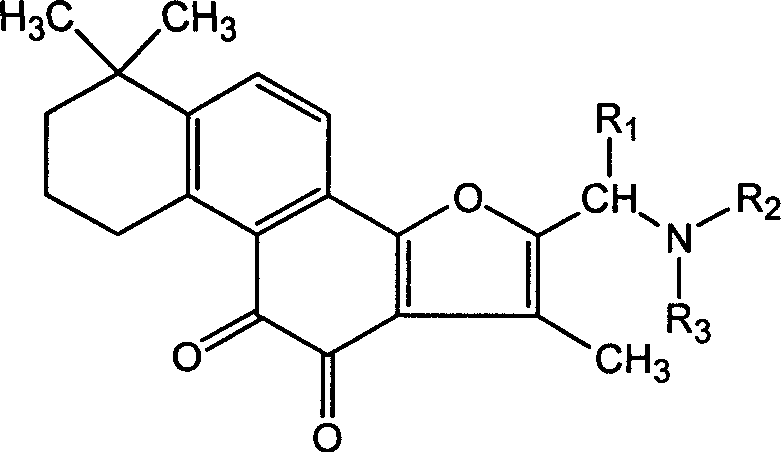
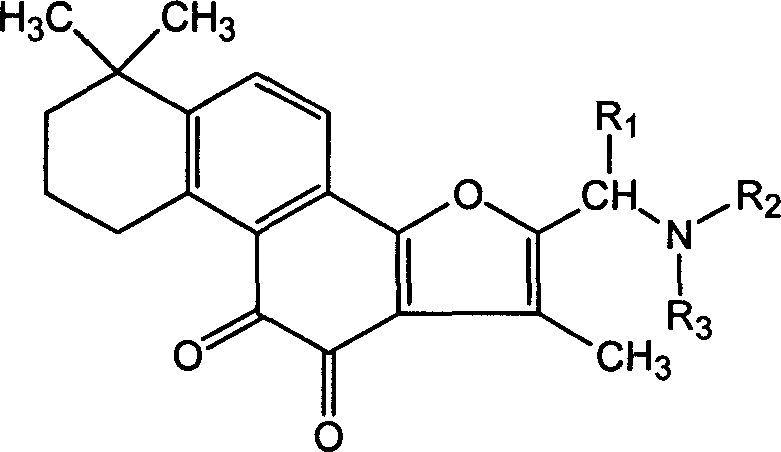
Tanshinone IIA derivatives and pharmaceutical application thereof
A derivative, tanshinone technology, applied in the field of medicine, can solve the problems of low pH value, high product irritation, failure to fully exert the medicinal value and social value of tanshinone IIA, and achieve the effect of increasing drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1. 2-(diallylamine) methyl-tanshinone II A synthesis steps and structural confirmation
[0053] Mix 1.56g of tanshinone, 0.5mL of 37% formaldehyde solution, 2.4g of diallylamine, and 50mL of acetic acid, heat and reflux in an oil bath for 5h, remove the solvent from the mixture under reduced pressure to obtain a red solid, dissolve it in water, filter, and adjust the filtrate with alkali pH to 9, filtered, the filter cake was dried, and separated by silica gel column chromatography (eluent: chloroform / methanol=15 / 1) to obtain 1.15 g (yield 53%) of the expected compound.
[0054] 1 H-NMR (CDCl 3)δ7.71(d, 1H), 7.66(d, 1H), 5.31~5.45(m, 2H), 4.65~4.81(m, 4H), 3.50(s, 2H), 3.15(m, 2H), 2.55 ~2.71(m, 4H), 2.18(s, 6H), 2.07(s, 3H), 1.74(m, 2H), 1.61(m, 2H).
Embodiment 2
[0055] Example 2. Synthesis steps and structural confirmation of 2-(sodium iminodiacetate) methyl-tanshinone II A
[0056] Mix 1.56g of tanshinone, 0.5mL of 37% formaldehyde solution, 4.2g of iminodiacetic acid hydrochloride, and 50mL of acetic acid, heat and reflux in an oil bath for 5h, remove the solvent from the mixture under reduced pressure to obtain a red solid, dissolve in water, filter, and filtrate Add alkali to adjust the pH to 9, filter, dry the filter cake, and separate by silica gel column chromatography (eluent: chloroform / methanol=15 / 1) to obtain 1.41 g (yield 60%) of 2-iminodiacetic acid-tanshinone II A, and then dissolved in twice the molar amount of sodium bicarbonate solution, filtered to remove insoluble matter, and the filtrate was dried to obtain the desired compound.
[0057] 1 H-NMR (CDCl3) δ7.62(d, 1H), 7.56(d, 1H), 3.64(s, 2H), 3.35~3.51(m, 4H), 3.15(m, 2H), 2.09(s, 6H) ), 1.95(s, 3H), 1.75(m, 2H), 1.61(m, 2H).
Embodiment 3
[0058] Example 3. 2-(cyclohexylamine) methyl-tanshinone II A synthesis steps and structure confirmation
[0059] Mix 1.56g of tanshinone, 0.5mL of 37% formaldehyde solution, 2.5g of cyclohexylamine, and 50mL of acetic acid, heat and reflux in an oil bath for 5 hours, remove the solvent from the mixture under reduced pressure to obtain a red solid, dissolve it in water, filter, and adjust the pH of the filtrate to 9. Filter, dry the filter cake, and separate by silica gel column chromatography (eluent: chloroform / methanol=15 / 1) to obtain 1.41 g (yield 60%) of the expected compound.
[0060] 1 H-NMR (CDCl3) δ7.68(d, 1H), 7.52(d, 1H), 3.55(s, 2H), 3.21~3.32(m, 4H), 3.13(m, 2H), 2.12(s, 6H) ), 2.03(s, 3H), 1.75(m, 2H), 1.62(m, 2H), 1.52~1.61(m, 4H), 1.32~1.41(m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com