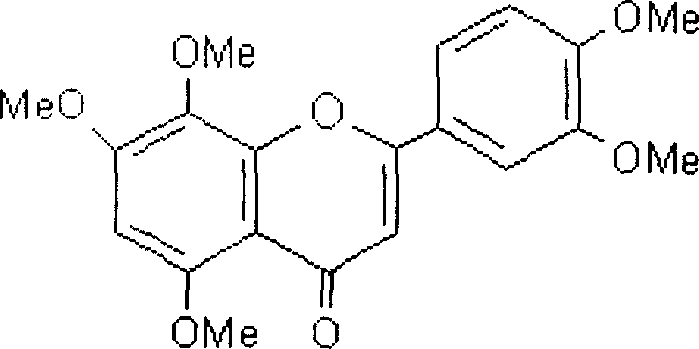
Polymethoxylated flavones and its preparation method
A polymethoxyflavonoid injection and the technology of polymethoxyflavonoids are applied in the directions of anti-inflammatory agents, pharmaceutical formulations, non-central analgesics, etc., and can solve the problems of poor stability, non-compliance with medicinal standards, and unsuitable for industrial production, etc. problem, to achieve the effect of good solubility and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Weigh 180 grams of polyoxyethylene hydrogenated castor oil (RH60) in an airtight container, add 400 ml of absolute ethanol, mix well at room temperature into a liquid state, then add nobiletin, tangeretin, sweet orange element, heptalysin 30 grams of the mixture of oxyflavone and isotangerine, after the container is sealed, make it completely dissolve in a water bath at 50°C. Remove and let stand at room temperature for 45 minutes. Then add activated carbon for needles with a total liquid weight of 0.1%, stir at room temperature for 20 minutes, filter with a No. 4 sand core filter rod, and filter with a 0.22μф300mm sterile membrane under pressure. After the predicted content and pyrogen are qualified, aseptically pack 1000 bottle. It can be used for intramuscular injection after diluting with 5ml of water for injection before use.
Embodiment 2
[0026] Weigh 600 grams of Tween-80 into a sealable container, add 1000 ml of absolute ethanol, mix well at room temperature into a liquid state, then add 100 grams of a mixture of nobiletin, tangeretin and sweet orange, and seal the container , 70 ℃ water bath to make it completely dissolved. Take it out and let it stand at room temperature for 60 minutes. Then add activated carbon for needles with 0.5% of the total weight of the liquid, stir at room temperature for 15 minutes, filter with a No. 4 sand core filter rod, and filter with a 0.22μф300mm sterile membrane under pressure. After the predicted content and pyrogen are qualified, aseptically pack 1000 bottle. Diluted with 100ml 0.9% sodium chloride injection or 5% glucose injection before use, it can be used for intravenous injection.
Embodiment 3
[0028] Take by weighing 400 grams of polyoxyethylene hydrogenated castor oil (RH40) in an airtight container, add 1,2-propanediol 1500 milliliters, mix into a liquid state at room temperature, then add the mixture of sweet orange element, heptamethoxyflavone 50 grams, after the container is sealed, dissolve it completely in a 35°C water bath. Take it out and let it stand at room temperature for 70 minutes. Then add activated carbon for needles with a total liquid weight of 0.3%, stir at room temperature for 30 minutes, filter with a No. 4 sand core filter rod, and filter with a 0.22μф300mm sterile membrane under pressure. After the predicted content and pyrogen are qualified, aseptically pack 1000 bottle. Diluted with 100ml 0.9% sodium chloride injection or 5% glucose injection before use, it can be used for intravenous injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com