Process for preparing 1,1'-spiro indan or derivatives thereof
A technology of spirodihydroindane and its derivatives, which is applied in the field of organic compound preparation chemistry, can solve problems such as product separation troubles, equipment corrosion, etc., and achieve the effects of low cost, less pollution, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
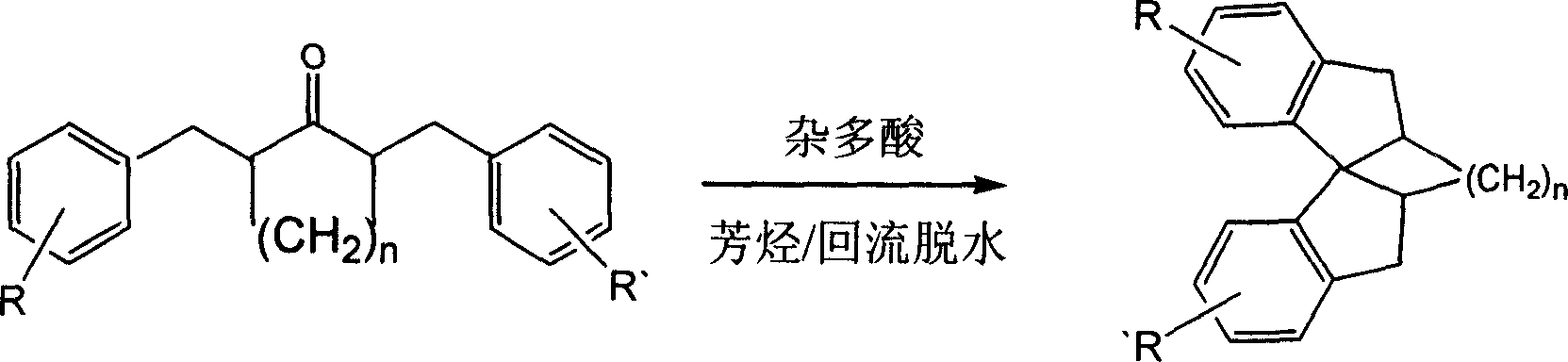
[0015] 4.8 g (20 mmol) of 1,5-diphenyl-3-pentanone, 3 g (1 mmol) of silicomolybdic acid and 60 ml of benzene were added to a flask. Install the water separator, reflux the condenser, heat and reflux for dehydration. After the reaction, the insoluble solid was removed by filtration and washed with chloroform, the organic phases were combined, and the solvent was evaporated. A light yellow liquid was obtained, which was purified by silica gel column chromatography to obtain 2.6 g of 1,1'-spirodihydroindene as a colorless liquid, with a yield of 60%. Proton NMR spectrum (300M, deuterium): 2.14-2.35 (m, 4H) 3.02 (t, J = 13Hz, 4H) 6.92 (d, J = 7Hz, 2H) 7.11-7.29 (m, 6H); infrared: 1602, 1455, 751 (cm -1 ).
[0016]
[0017] 1,5-Diphenyl-3-pentanone 1,1'-spirodihydroindane
Embodiment 2
[0019] 4.8 g (20 mmol) of 1,5-diphenyl-3-pentanone, 14 g (5 mmol) of phosphomolybdic acid and 60 ml of toluene were added to a flask. Install the water separator, reflux the condenser, heat and reflux for dehydration. After the reaction, the insoluble solid was removed by filtration and washed with chloroform, the chloroform and toluene were combined, and the solvent was removed by rotary evaporation. A pale yellow liquid was obtained, which was purified by silica gel column chromatography. 1,1'-spirodihydroindane was obtained as 4 g of colorless liquid with a yield of 91%. Proton NMR spectrum (300M, deuterium): 2.14-2.35 (m, 4H) 3.02 (t, J = 13Hz, 4H) 6.92 (d, J = 7Hz, 2H) 7.11-7.29 (m, 6H); infrared: 1602, 1455, 751 (cm -1 ).
Embodiment 3
[0021] Add 5.3 g (20 mmol) of 2,5-dibenzylcyclopentanone, 14.4 g (5 mmol) of silicotungstic acid and 60 ml of toluene into a flask. Install the water separator, reflux the condenser, heat and reflux for dehydration. After the reaction, the insoluble solid was removed by filtration and washed with chloroform, the chloroform and toluene were combined, and the solvent was removed by rotary evaporation. A light yellow solid was obtained, which was recrystallized from methanol. 2,2'-Ethylene-1,1'-spirodihydroindene was obtained as 3.8 g of a colorless solid with a melting point of 100-102°C and a yield of 77%.
[0022]
[0023] 2,5-Dibenzylcyclopentanone 2,2'-Ethylene-1,1'-spirodihydroindane
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com