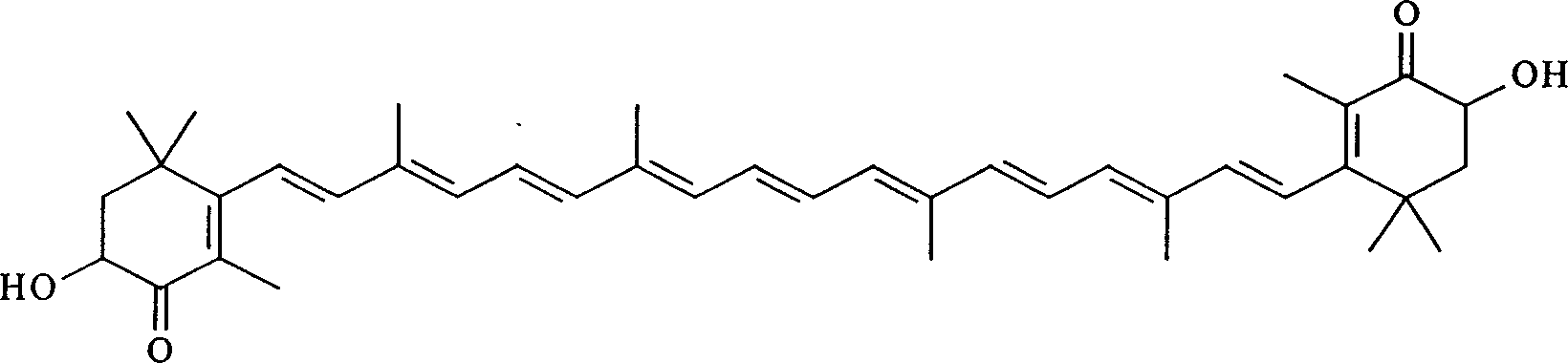
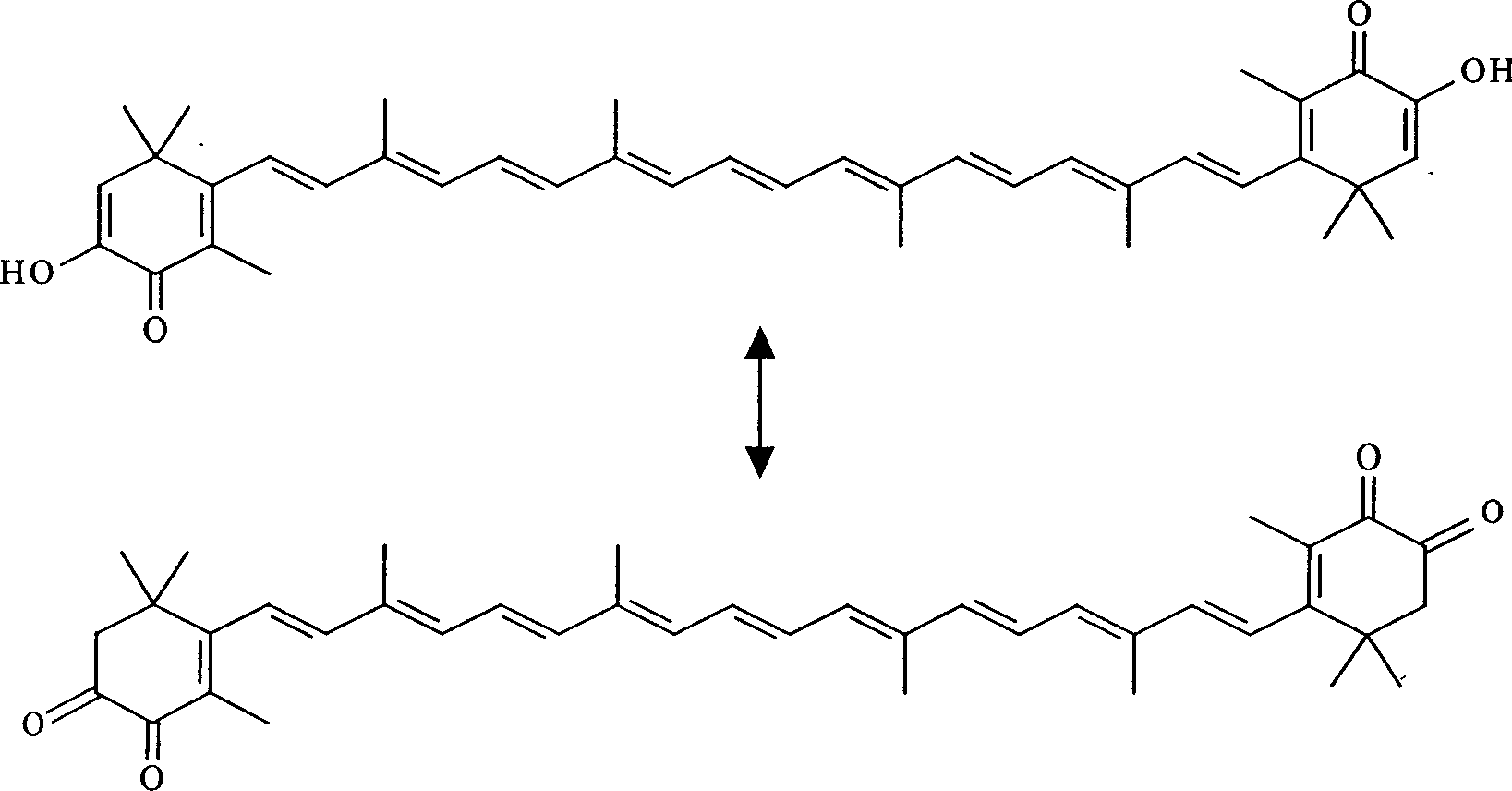
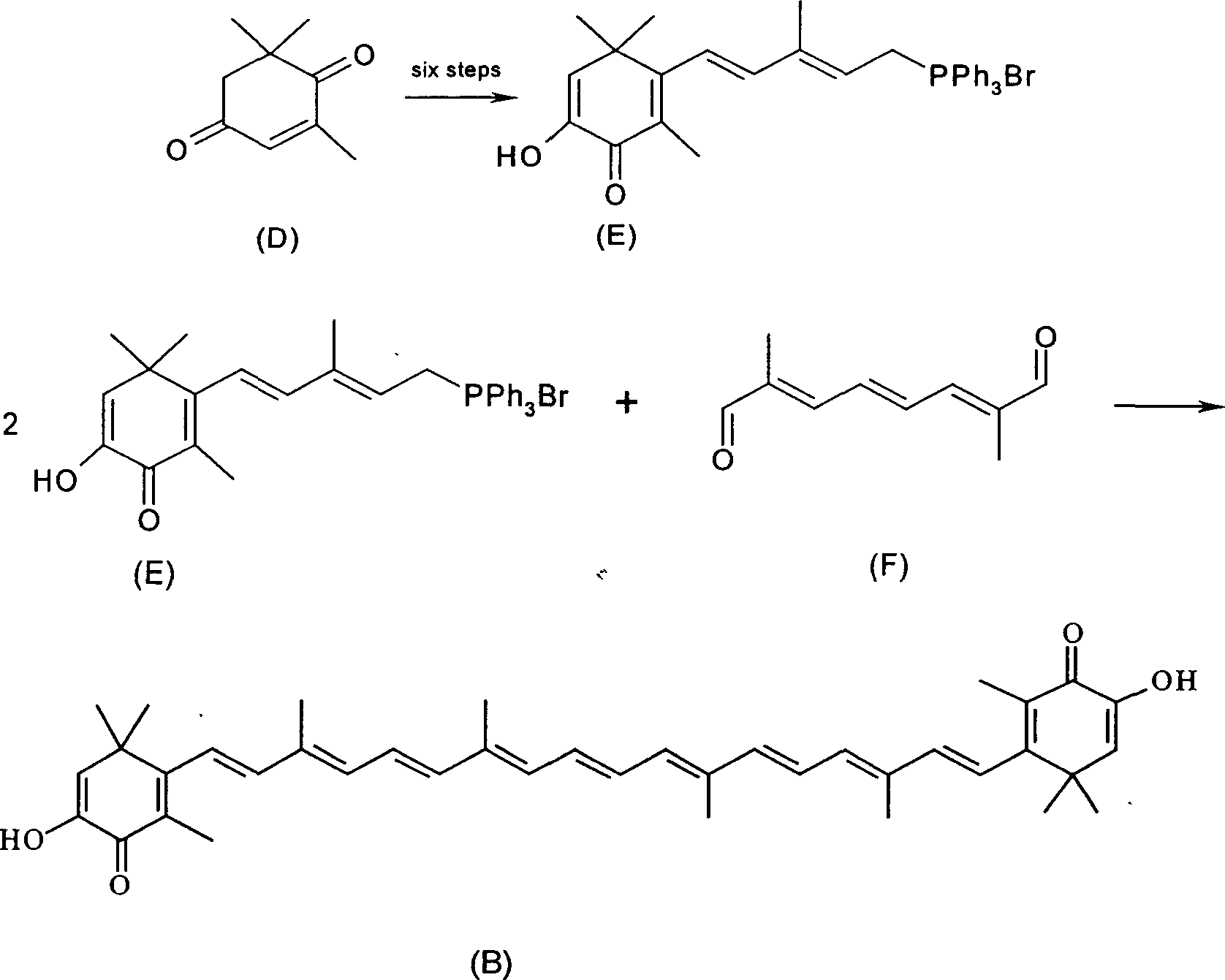
Synthesis of astaxanthin
A technology of astaxanthin and astaxanthin, applied in the direction of organic chemistry, can solve the problems of less than 30% total yield, many chemical reagents, complicated operation, etc., and achieves convenient operation, good reaction selectivity and simple process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 3.00g of astaxanthin (A) and 1000ml of methanol into a 1000ml three-neck flask, then add 3.26g of sodium methoxide according to 12 times the molar number of (A), and feed oxygen at a rate of 0.15L / min under stirring to control the reaction temperature temperature was 65°C, and the temperature was kept for 17 hours. After the reaction is completed, the solvent is removed to obtain a solid crude product, which is then dissolved with dichloromethane, filtered, and the filtrate is precipitated to obtain 0.88 g of unreacted astaxanthin (A), and then the residual solid is dissolved in water to obtain an alkaline reaction solution. Neutralize until the pH value becomes 4, stop adding acid, filter, wash and dry to obtain 2.04g of product, which contains 81.2% astaxanthin (B), and the actual yield is 78.7%.
Embodiment 2
[0032] Add 2.00g of astaxanthin (A) and 1000ml of ethanol into a 1000ml three-neck flask, then add 1.50g of potassium hydroxide according to 8 times the molar number of (A), and feed oxygen at a rate of 0.3L / min under stirring to control the reaction The temperature was 55°C, and the reaction was kept for 11 hours. After the reaction is completed, the solvent is removed to obtain a solid crude product, which is then dissolved with dichloromethane, filtered, and the filtrate is precipitated to obtain 0.43 g of unreacted astaxanthin (A), and then the residual solid is dissolved in water to obtain an alkaline reaction solution. Neutralize until the pH value becomes 5, stop adding acid, filter, wash and dry to obtain 1.53g of product, which contains 90.1% astaxanthin (B), and the actual yield is 88.4%.
Embodiment 3
[0034] Add 4.50g of astaxanthin (A) and 1000ml of isopropanol in a 1000ml three-necked flask, then add 7.61g of potassium hydroxide by 18 times the molar number of (A), and feed oxygen at a rate of 0.08L / min under stirring, Control the reaction temperature to 75° C., and keep the reaction for 28 hours. After the reaction is completed, the solvent is removed to obtain a solid crude product, which is then dissolved with dichloromethane, filtered, and the filtrate is precipitated to obtain 1.82 g of unreacted astaxanthin (A), and then the residual solid is dissolved in water to obtain an alkaline reaction solution. Neutralize until the pH value becomes 4, stop adding acid, filter, wash and dry to obtain 2.43g of product, which contains 71.2% astaxanthin (B), and the actual yield is 64.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com