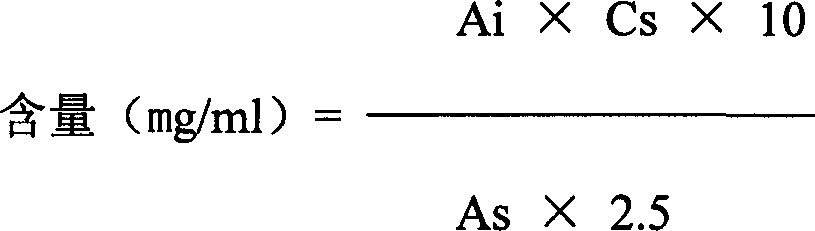Quality control method of spirit quieting oral liquid preparation
A quality control method and technology of liquid preparations, applied in the direction of testing pharmaceutical preparations, measuring devices, pharmaceutical formulations, etc., can solve problems such as difficult to achieve quality, difficult to control the quality of agents, and incomplete methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] The quality control of embodiment 1 tranquilizing syrup
[0118] Take 50g of Chinese herbal medicine Ganoderma lucidum, 100g of Atractylodes macrocephala (stir-fried), 100g of Ligustrum lucidum (made), 150g of Albizia radix, 150g of Shouwu vine, 250g of agrimony, 100g of Eclipta chinensis, and 50g of licorice. , 5 hours for the first time, 3 hours for the second time, combine the decoction, filter, concentrate the filtrate to a relative density of 1.05 (90°C), let it stand for 24 hours, take the supernatant for later use; Boil twice, the first time for 2 hours, the second time for 1.5 hours, combine the decoction, filter, and concentrate the filtrate to a relative density of 1.05 (90°C), let it stand for 24 hours, and take the supernatant for later use. Take another 264g of sucrose, add an appropriate amount of water, boil to dissolve, filter, mix with the above two concentrates, continue to concentrate to a relative density of 1.10 (90°C), let cool, add 3g of sodium be...
Embodiment 2
[0143] The quality control of embodiment 2 Anshen Oral Liquid
[0144] Take 50g of Chinese herbal medicine Ganoderma lucidum, 100g of Atractylodes macrocephala (stir-fried), 100g of Ligustrum lucidum (made), 150g of Albizia radix, 150g of Shouwu vine, 250g of agrimony, 100g of Eclipta chinensis, and 50g of licorice. , 5 hours for the first time, 3 hours for the second time, combine the decoction, filter, concentrate the filtrate to a relative density of 1.05 (90°C), let it stand for 24 hours, take the supernatant for later use; Boil twice, the first time for 2 hours, the second time for 1.5 hours, combine the decoction, filter, and concentrate the filtrate to a relative density of 1.05 (90°C), let it stand for 24 hours, and take the supernatant for later use. Add 264ml of simple syrup, 2.5g of sodium benzoate, mix with the above two concentrates, add water to 1000ml, stir well, filter, potting, each bag has a capacity of 10ml, and you get it.
[0145] 【Properties】This product...
Embodiment 3
[0170] Take 50g of Ganoderma lucidum, 100g of Atractylodes macrocephala (stir-fried), 100g of Ligustrum lucidum (made), 150g of Albizia juniper, 150g of Shouwu vine, 250g of agrimony, 100g of Eclipta, 50g of licorice,
[0171] [preparation method], [properties], [inspection] are all identical with embodiment 1. Its quality control methods are:
[0172] 1, measure the content of glycyrrhizic acid monoammonium salt with high performance liquid chromatography (an appendix VI D of Chinese Pharmacopoeia edition in 2000), can carry out according to the following steps:
[0173] a. Chromatographic conditions: use octadecylsilane bonded silica gel as filler; Mobile phase is acetonitrile-2.5% glacial acetic acid aqueous solution (26:58); Detection wavelength is 247nm; The calculation should be no less than 2000.
[0174] b. Preparation of reference substance solution Take about 10 mg of glycyrrhizinate monoammonium salt reference substance, accurately weighed, put in a 50ml measuring...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com