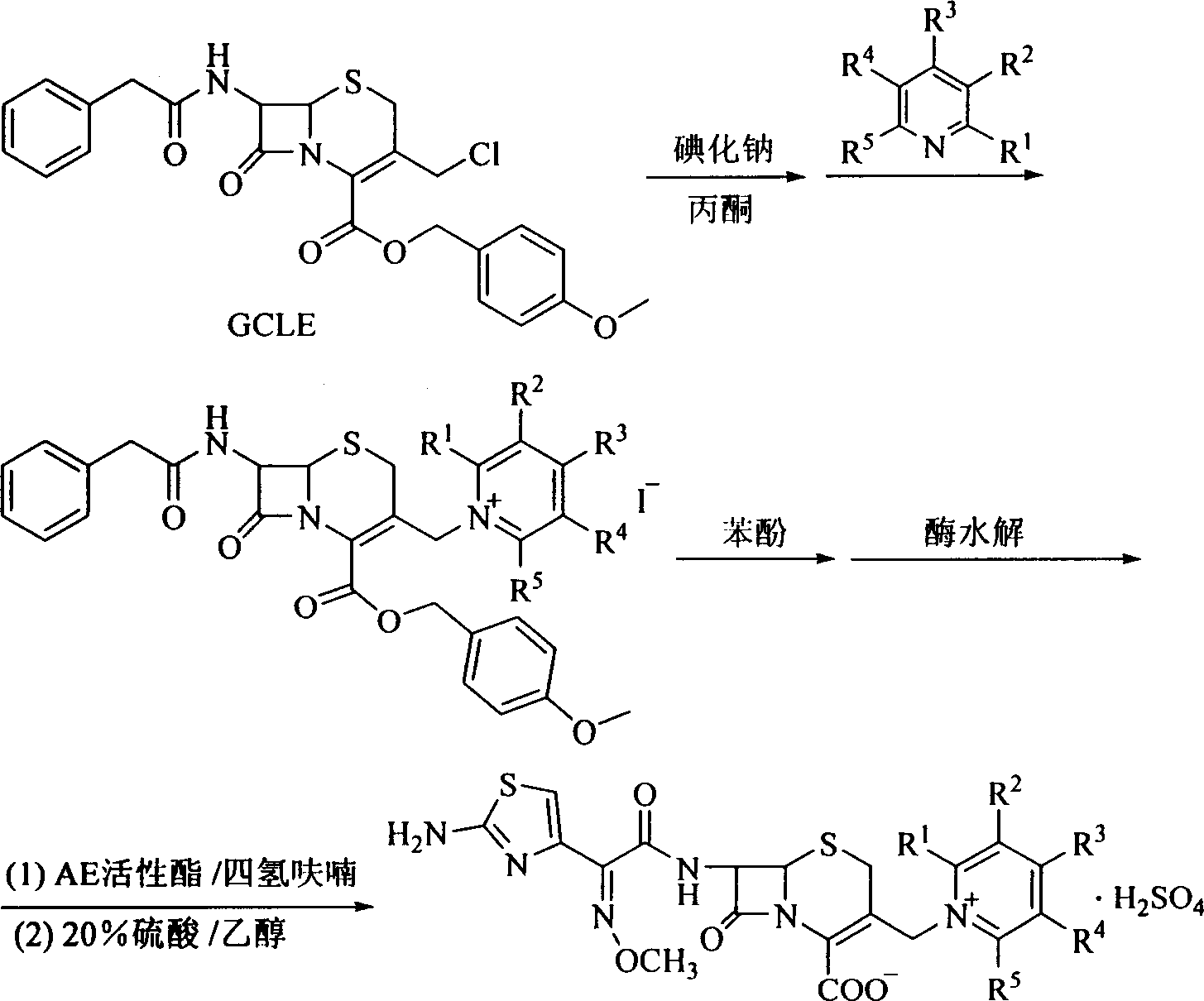
Synthesis process of cefpirome and its analog
A technology of cefpirome and its analogues, which is applied in the field of new synthetic technology of the fourth-generation cephalosporin antibiotic cefpirome and its analogues, which can solve the problems of low yield, limited application prospects, harsh reaction conditions, etc. , to achieve the effect of cheap iodination reagent, simple synthesis process route and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The synthesis of embodiment 1, cefpirome:
[0021] (1), mix 102g of sodium iodide and 2L of acetone together and stir evenly, add 167g of GCLE at 0°C, and monitor the reaction process with high performance liquid chromatography;
[0022] (2), after the reaction is complete, the reaction solution is poured into a separatory funnel, ethyl acetate and water are added, and the liquid is separated, and the organic layer is washed with an aqueous solution of sodium thiosulfate and saturated brine, dried over anhydrous sodium sulfate, and filtered;
[0023] (3), stir the filtrate, and slowly add 163 g of 2,3-cyclopentenopyridine ethyl acetate solution dropwise at -25°C, after the dropwise addition, continue the reaction, and monitor the reaction process with high performance liquid chromatography;
[0024] (4), after the reaction is complete, filter, the filter cake is rinsed with ethyl acetate, and vacuum-dried to obtain 180 g of a light yellow solid;
[0025] (5), above-men...
Embodiment 2
[0029] Embodiment 2, the synthesis of cefpirome analogue:
[0030] (1) Mix 120g of sodium iodide and 2L of acetone and stir evenly, add 195g of GCLE at 0°C, and monitor the reaction process with high performance liquid chromatography;
[0031] (2), after the reaction is complete, the reaction solution is poured into a separatory funnel, ethyl acetate and water are added, and the liquid is separated, and the organic layer is washed with an aqueous solution of sodium thiosulfate and saturated brine, dried over anhydrous sodium sulfate, and filtered;
[0032] (3), stirring the filtrate, slowly dripping an ethyl acetate solution of 126 g of pyridine at -5° C., after the dropwise addition, continue the reaction, and monitor the reaction process with high performance liquid chromatography;
[0033] (4), after the reaction is complete, filter, the filter cake is rinsed with ethyl acetate, and vacuum-dried to obtain 216 g of a light yellow solid;
[0034] (5), above-mentioned light y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com