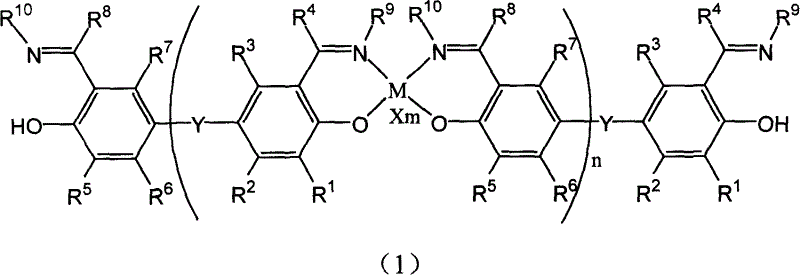
Pretransistion metal catalytic system for ethene polymerisation and copolymerisation, its preparation method and uses
A technology for pre-transition metal and ethylene polymerization, which is applied in the field of pre-transition metal catalytic systems, can solve problems such as not being retrieved, and achieve the effects of reducing costs and improving the catalytic activity of ethylene polymerization and copolymerization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Complex (L1) 3 Zr 2 Cl 4 Synthesis
[0046] 1.4, the synthesis of 4'-isopropylidene-bis(2-tert-butyl-phenol)
[0047] In a 500ml three-neck flask, add 138ml (0.6mol) of o-tert-butylphenol and 21.9ml (0.2mol) of acetone, stir, add 0.9ml of dodecanethiol, pass in hydrogen chloride gas, and react at room temperature for two days to obtain orange Viscous liquid, add 150ml of anhydrous ether, make it completely dissolved, then add 16.2g of NaHCO 3 Aqueous solution, the solution is light pink, then add an appropriate amount of distilled water to wash, collect the organic phase, add Na 2 SO 4 dry. Filter out Na 2 SO 4 , the filtrate was distilled under reduced pressure, the residue was added an appropriate amount of heptane, stirred to cool down, a large amount of white precipitate was precipitated, collected and dried to obtain white 4,4'-isopropylidene-bis(2-tert-butyl-phenol) solid powder.
[0048] Synthesis of 2.5,5'-isopropylidene-bis(3-tert-butyl-2-hydroxybenza...
Embodiment 2
[0071] Embodiment 2, complex (L2) 3 Zr 2 Cl 4 Synthesis
[0072] 1. Synthetic Ligand L2
[0073] Under a nitrogen atmosphere, in a 250ml there-necked flask, add 2.0g (5.05mmol) of 5,5'-isopropylidene-bis(3-tert-butyl-2-hydroxybenzaldehyde) synthesized by the method in Example 1, and use 60ml of methanol was dissolved, then 1.39ml (12.12mmol) of cyclohexylamine and 0.6ml of formic acid were added, and the reaction was stirred at room temperature for 24 hours. The precipitate was filtered off and dried in vacuo to obtain 0.7 g (1.25 mmol, 24.8% yield) of Ligand L2 as a yellow powder.
[0074] Its structural formula is as follows:
[0075]
[0076] Ligand L2
[0077] CI-mass spectrum: 558M +
[0078] 2. Synthesis of metal complexes (L2) 3 Zr 2 Cl 4
[0079] Under nitrogen atmosphere, add 1.07g (1.92mmol) of ligand L2 synthesized above into the three-necked flask, add 50ml of tetrahydrofuran to dissolve, then cool down to below -70°C, slowly add ...
Embodiment 3
[0083] Synthesis of metal complexes (L1) 9 Zr 8 Cl 16
[0084] Under a nitrogen atmosphere, add 0.63g (1.15mmol) of ligand L1 synthesized according to the method in Example 1 into the three-neck flask, add 25ml of tetrahydrofuran to dissolve, then cool down to below -70°C, and slowly add 1.5ml (2.42mmol) of n-butyl Lithium-based solution, react at this temperature for 1 hour, slowly warm up to room temperature, and react for 4 hours; transfer this solution to a constant pressure dropper, and slowly add it dropwise until 0.44g (1.15mmol) of the solution is dissolved below -70°C ZrCl 4 (THF) 2 In the tetrahydrofuran solution of 20ml, after dripping, rise to room temperature gradually, then react for about 18 hours, then, reflux reaction for 5 hours again; Distill under reduced pressure, after evaporating to dryness, dissolve with 40ml dichloromethane, filter out insoluble matter, The filtrate was evaporated to dryness under reduced pressure, washed with n-heptane, and sucke...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com