New brand of Aripipazole, and preparation method
A technology of aripiprazole and aripiprazole is applied in the application field of preparing medicine for treating schizophrenia, and can solve the problems of increased cost, complicated process, unfavorable industrialized production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
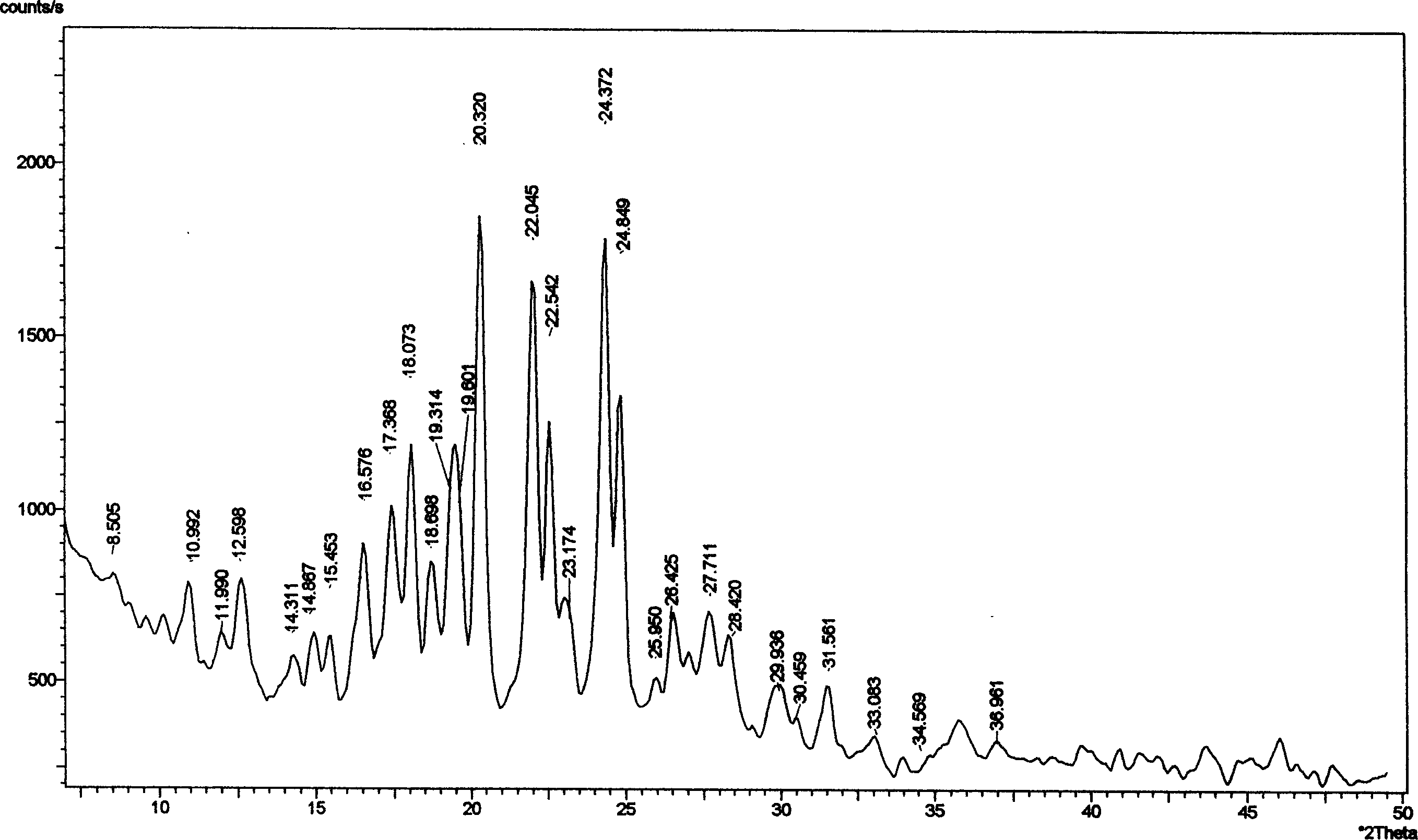
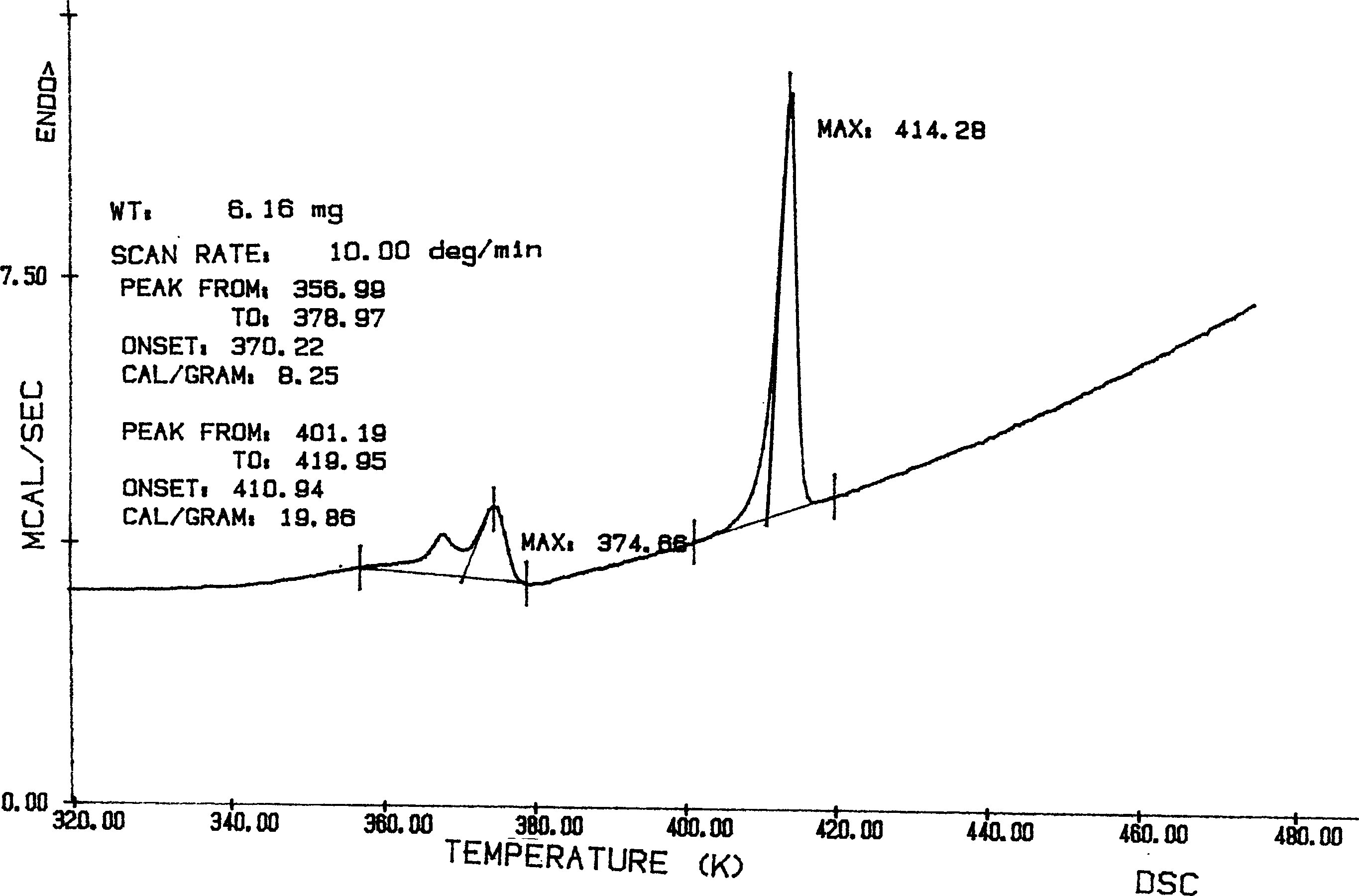
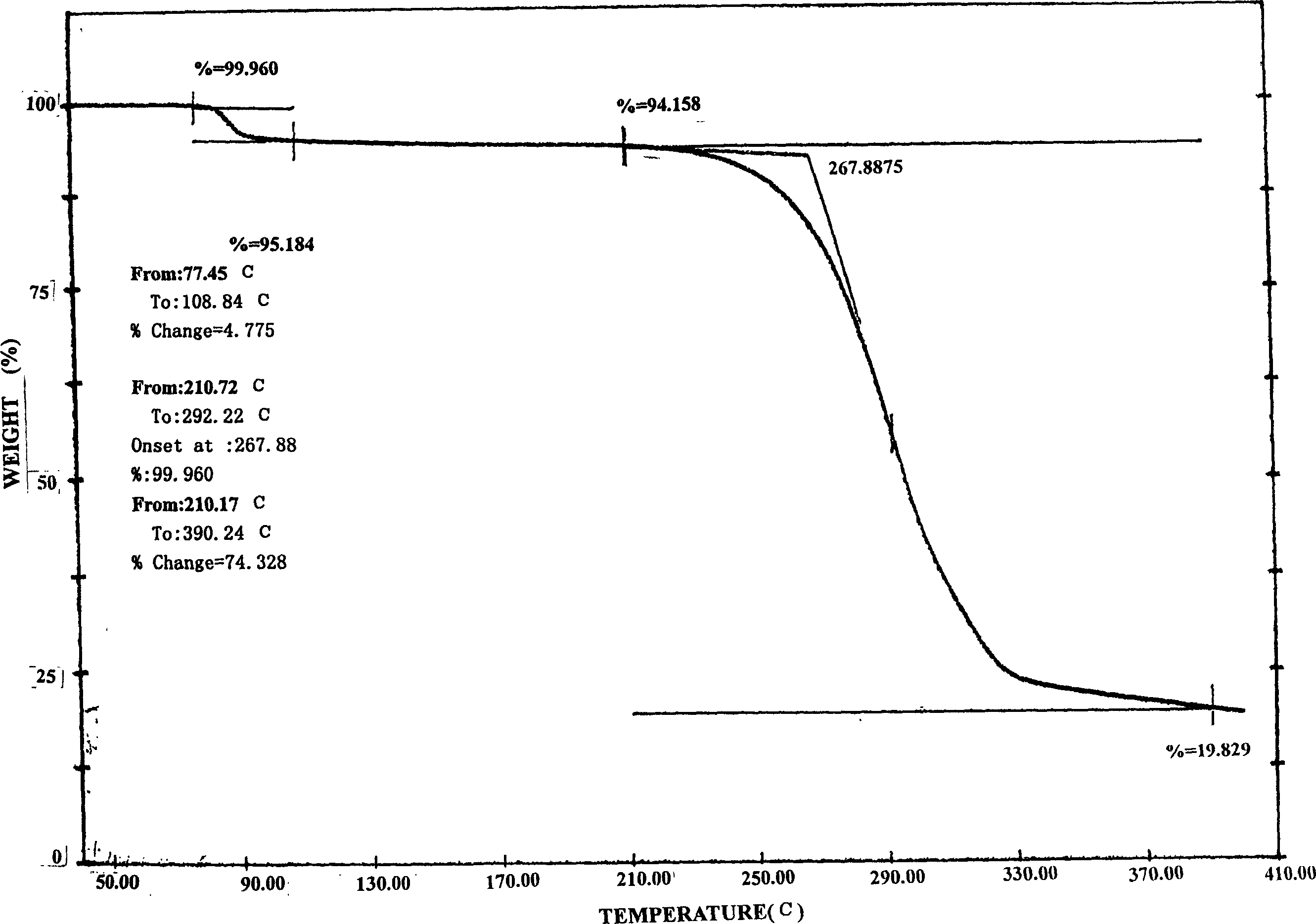
Image
Examples
Embodiment 1
[0059] Add 10g of aripiprazole and 150ml of absolute ethanol into the three-port reaction with a reflux condenser, heat to reflux with stirring, and stop heating and stirring after the aripiprazole is completely dissolved. Cool the crystallization system to 50°C naturally, keep it warm at 50°C for 3 hours, let it cool down to room temperature naturally, crystallize at room temperature for 24 hours, freeze and crystallize at -4°C for 24 hours, suction filter, wash, and The obtained crystals were placed in a desiccator at 50°C, and dried under normal pressure for 24 hours to obtain 9.0 g of flaky aripiprazole crystals, yield: 90%, mp: 137.0-138.0°C.
Embodiment 2
[0061] Add 10g of aripiprazole and 120ml of absolute ethanol into the three-port reaction with a reflux condenser, heat to reflux under stirring, and stop heating and stirring after the aripiprazole is completely dissolved. Cool the crystallization system to room temperature naturally, crystallize at room temperature for 36 hours, freeze and crystallize at -10°C for 8 hours, suction filter, wash, place the obtained crystals in a desiccator at 60°C, and dry at normal pressure for 8 hours 9.3 g of flaky aripiprazole crystals were obtained, yield: 93%, mp: 137.5-138.5°C.
Embodiment 3
[0063] Add 10g of aripiprazole and 100ml of absolute ethanol into the three-port reaction with a reflux condenser, and heat to reflux under stirring. After the aripiprazole is completely dissolved, quickly drop 50ml of tetrahydrofuran into the crystallization system under stirring, about The dropwise addition was completed in 5 minutes, and after the stirring was even, stop heating and stirring. The crystallization system was naturally lowered to 60°C for 6 hours, then allowed to drop to 40°C, kept at 40°C for 2 hours, and finally allowed to cool down to room temperature, crystallized at room temperature for 6 hours, then frozen at -5°C After 18 hours of crystallization, suction filtration, washing, the obtained crystals were placed in a dryer at 75°C, and dried under normal pressure for 6 hours to obtain 8.8 g of flaky aripiprazole crystals, yield: 88%, mp: 137.0~ 138.0°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com