Storage-stable and bio-stable formulations of ACE inhibitors, and methods for preparation thereof
A storage stability and inhibitor technology, which can be used in medical preparations containing active ingredients, pharmaceutical formulations, active ingredients of heterocyclic compounds, etc., and can solve problems such as stability that are not discussed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
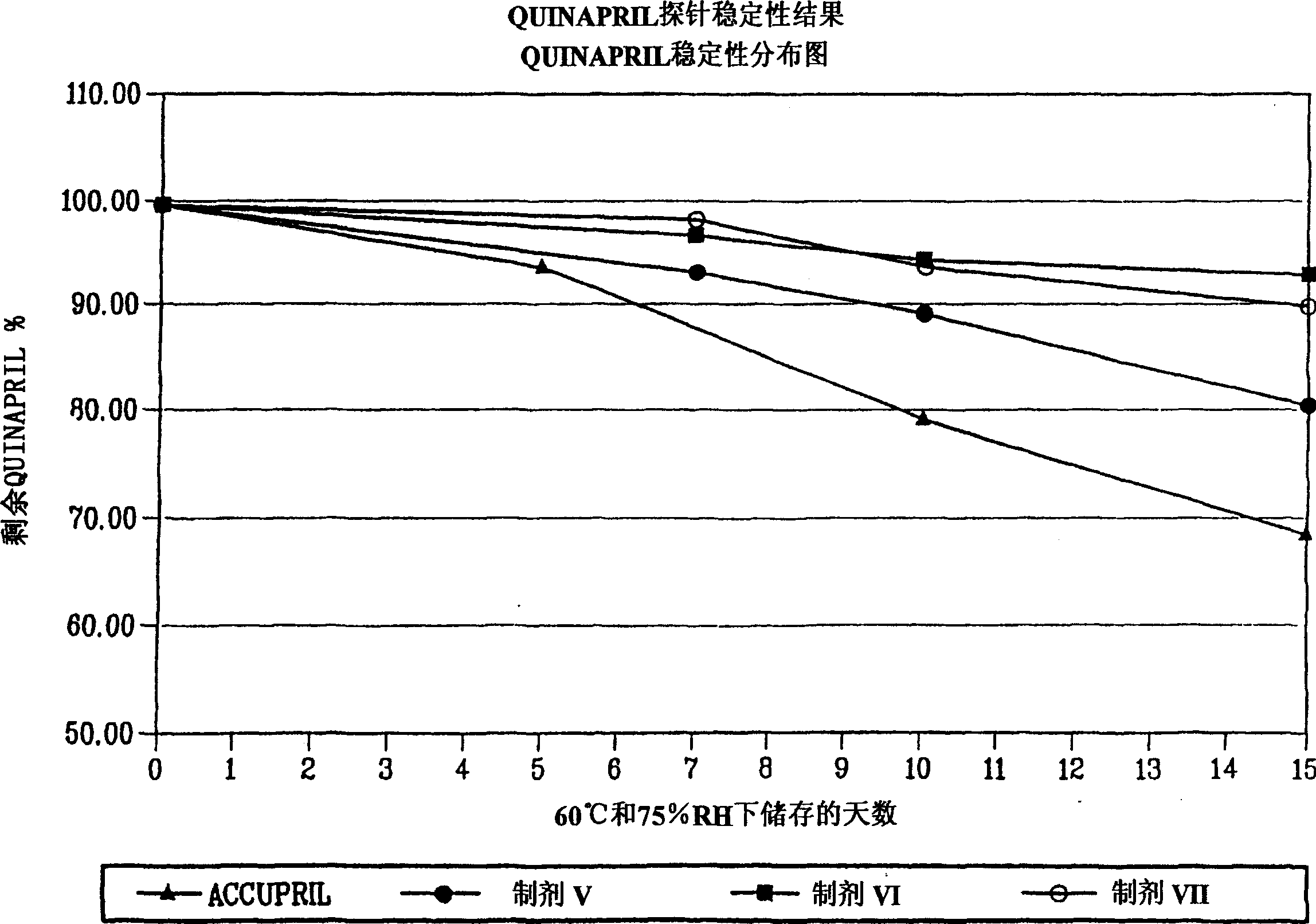
[0034] The invention provides a storage-stable and biologically stable ACE inhibitor, especially a preparation method of enalapril maleate and quinapril hydrochloride preparations. The method comprises the steps of: mixing an ACE inhibitor, such as enalapril maleate, with alcohol to form an alcohol dispersion of the ACE inhibitor, and then dissolving or dispersing the metal compound in water to form a metal compound solution or dispersion, The alcoholic dispersion of the ACE inhibitor and the aqueous solution or dispersion of the metal compound are mixed together to form a "final aqueous alcoholic solution or dispersion" of the ACE inhibitor and the metal compound. In another embodiment, the method comprises the steps of: mixing an ACE inhibitor, such as quinapril hydrochloride, with a mixture of alcohol and water (aqueous alcoholic system) to form an aqueous alcoholic dispersion of the ACE inhibitor, and The metal compound is added thereto under constant stirring to form a "f...
Embodiment 1
[0059] The amounts of ingredients provided in the formulations below are by equivalent weight (mg per unit dose (mg / ud)). The approximate batch size is about 6000 units.
[0060] Preparation of Formulation I
[0061] Enalapril maleate (20 mg / ud; Byron Chem. Co., Long Island City, NY) was suspended in SD3A denatured alcohol (50 mg / ud) with stirring at 500 rpm. In less than 10 seconds, enalapril maleate was completely dispersed in alcohol. In a separate container, sodium bicarbonate (11 mg / ud) and povidone (polyvinylpyrrolidone; Plasdone(R), ISP, Bound Brook, New Jersey) were dissolved in 100 mg / ud purified water (USP). The sodium bicarbonate / povidone solution was gradually added to the alcohol dispersion of the drug under constant stirring (200 rpm) until a transparent solution was obtained, resulting in Solution 1, for example, the solution was free of foam (bubbles).
[0062] Microcrystalline cellulose (225 mg / ud, Avicel® PH200; FMC Corporation, Philadelphia, PA), sodium s...
Embodiment 2
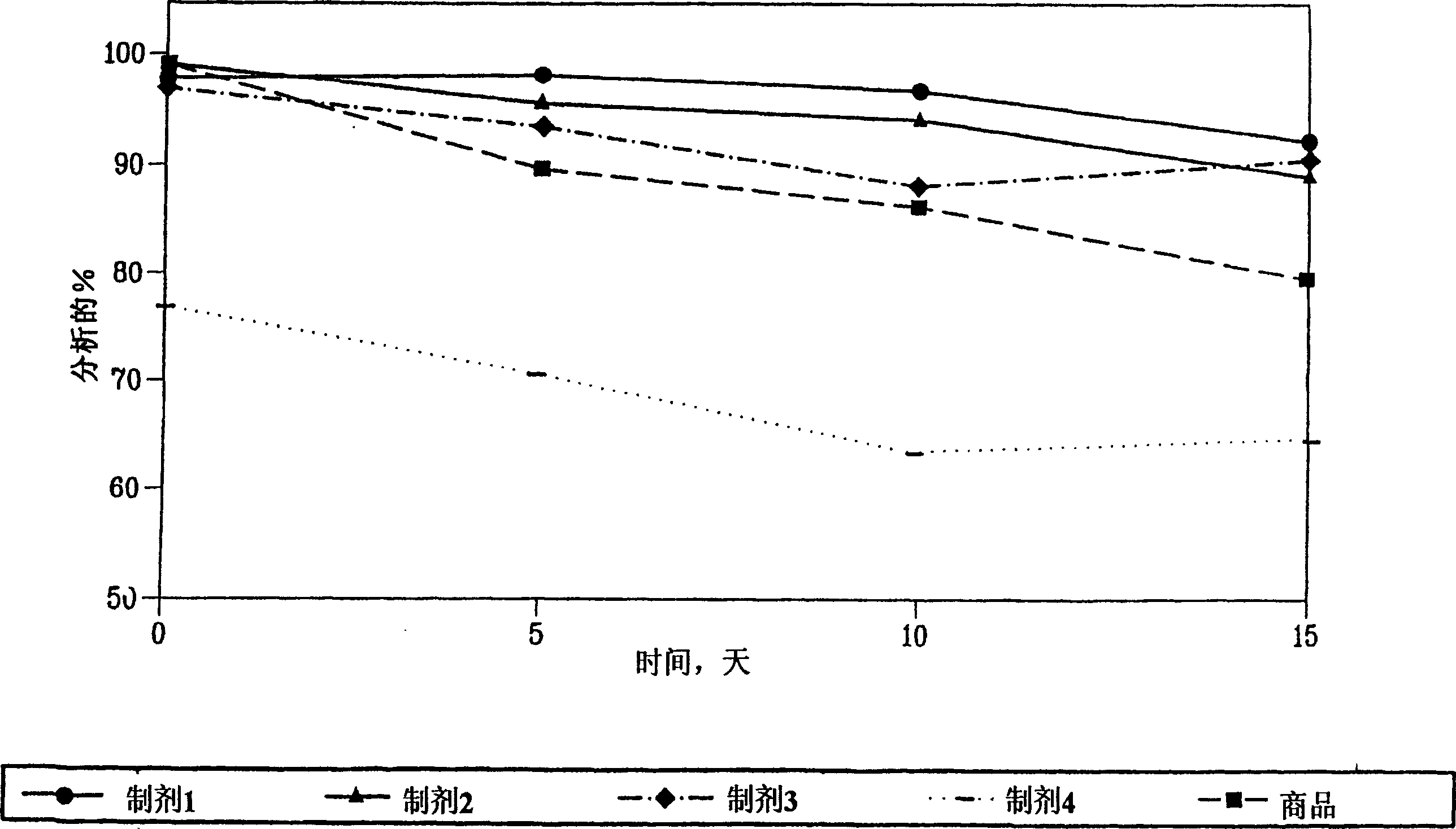
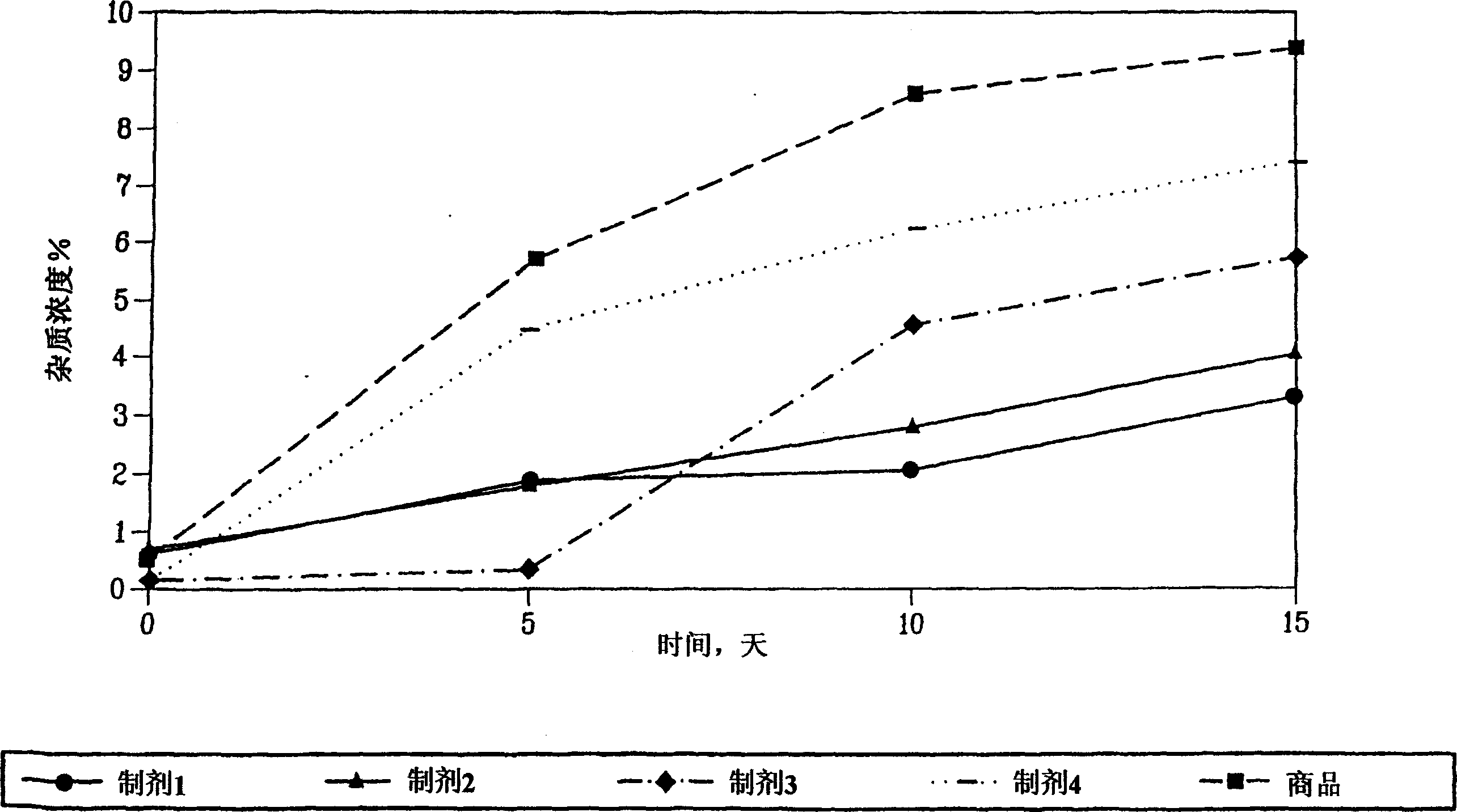
[0121] Comparison of stability profiles of different formulations of enalapril maleate.
[0122] Comparison of the stability profiles of different formulations of enalapril maleate. Formulation stability of stabilized enalapril maleate (Formulations I-IV, as described above) and a marketed formulation of enalapril maleate, known as "enalapril- Commodity" VASOTEC TM (Merck & Co.) for comparison. Formulations were stored at 60°C and 75% relative humidity to simulate long-term storage. Formulation stability was assessed by HPLC at 5, 10, and 15 days.
[0123] like figure 1 As shown, at the 5, 10, and 15 day time points, formulation I was more effective than VASOTEC TM Formulations and Formulations II-IV are more stable. At the 5 and 10 day time points, Formulation II exhibited greater TM Better stability of formulations. Formulation II was more effective than VASOTEC at the 5, 10, and 15 day time points TM Formulation and Formulation IV are more stable.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com