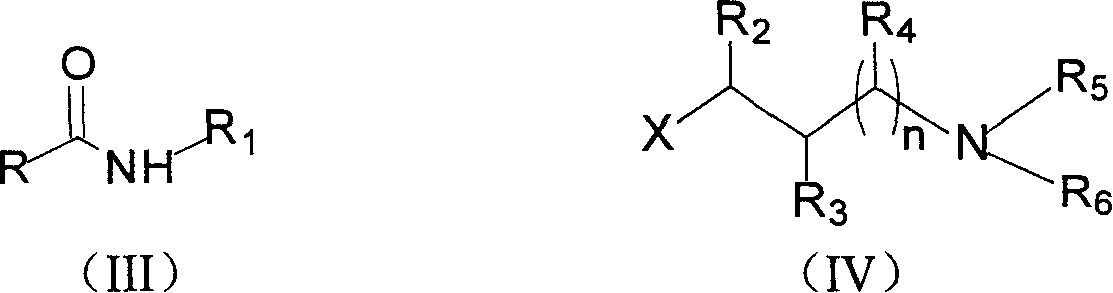Anti-hepatitis B virus tertiary amine oxide and preparation method and uses for manufacturing drug
A tertiary amine oxide and anti-hepatitis B technology, which is applied in antiviral agents, drug combinations, medical preparations containing active ingredients, etc., can solve the problems of expensive drugs and achieve low preparation costs and simple synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: the synthesis of N-phenyl-N-(3-dimethylaminopropyl) acetamide
[0058] Take 15mL DMSO, weigh 0.500g acetanilide, add to a 50mL round bottom flask, add 0.279g70% sodium hydride in batches, stir at room temperature for 0.5 hours, then add 0.704g N,N-dimethylamino-3-chloropropane salt The acid salt was added to the above mixture under vigorous stirring. After stirring at room temperature for 1 hour, the reaction system was heated in an oil bath, and the temperature was slowly raised to 70 degrees. After 5 hours, it was cooled to room temperature, and 100 mL of water was added to the reaction system. Extract with methane, combine the organic layers, wash the organic layer several times with water, dry the organic layer with anhydrous sodium sulfate sufficiently, remove the solvent under reduced pressure to obtain a crude product, dissolve the crude product in 10% dilute hydrochloric acid, wash with ethyl acetate, discard The ethyl acetate layer and the aqueous...
Embodiment 2
[0059] Embodiment 2: the synthesis of N-(3-dimethylaminopropyl) cyclocaprolactam
[0060] Take 15mL DMSO, weigh 2.000g cyclocaprolactam, add it to a 50mL round bottom flask, add 0.934g70% sodium hydride in batches, stir at room temperature for 0.5 hours, then add 3.356g N,N-dimethylamino-3-chloropropane salt The acid salt was added to the above mixture under vigorous stirring. After stirring at room temperature for 1 hour, the reaction system was heated in an oil bath, and the temperature was slowly raised to 80 degrees. After 8 hours, it was cooled to room temperature, and 100 mL of water was added to the reaction system. Extract with methane, combine the organic layers, wash the organic layer several times with water, dry the organic layer with anhydrous sodium sulfate sufficiently, remove the solvent under reduced pressure to obtain a crude product, dissolve the crude product in 10% dilute hydrochloric acid, wash with ethyl acetate, discard The ethyl acetate layer and the a...
Embodiment 3
[0061] Embodiment 3: the synthesis of N-n-butyl-N-(3-dimethylaminopropyl) acetamide
[0062] Take 15mL DMSO, weigh 2.000g N-n-butylacetamide, add it to a 50mL round bottom flask, add 1.282g70% sodium hydride, stir at room temperature for 0.5 hours, then add 3.223g N, N-dimethylamino-3- Chloropropane hydrochloride was added to the above mixture under vigorous stirring. After stirring at room temperature for 1 hour, the reaction system was heated in an oil bath, and the temperature was slowly raised to 60 degrees. After 9 hours, it was cooled to room temperature, and 100 mL of water was added to the reaction system. Extract with dichloromethane, combine the organic layers, wash the organic layer with water for several times, and dry thoroughly with anhydrous sodium sulfate, remove the solvent under reduced pressure to obtain a crude product, dissolve the crude product in 10% dilute hydrochloric acid, and wash with ethyl acetate , discard the ethyl acetate layer, adjust the pH of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com