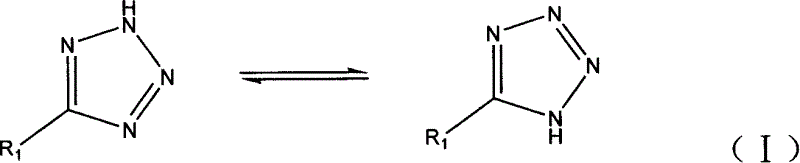
Chemical synthesis method of 1,2,3,4-tetra nitroazole kind compound
A technology of chemical synthesis and tetrazole, which is applied in the field of 1, can solve the problems of high reaction temperature and low yield, and achieve the effects of high reaction yield, low dosage and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The molar ratio of nitrile, sodium azide, and zinc trifluoromethanesulfonate is 1:1:0.1, the nitrile is benzonitrile, and the water consumption is 10 times of the nitrile mass.
[0035] In a 150ml four-necked flask equipped with a thermometer, reflux condenser and mechanical stirring, add 100g of water, 10.3g (0.1mol) of benzonitrile, 6.5g (0.1mol) of sodium azide, and 3.63g of zinc trifluoromethanesulfonate (10mmol), heated to reflux (100°C), and refluxed for 4 hours. After the reaction was completed, it was cooled to room temperature and neutralized to pH=5 with concentrated hydrochloric acid. Filter and collect the precipitated white solid to obtain 13.4 g of 5-phenyl-1H-tetrazolium with a yield of 90%, a purity of 98.2%, and a melting point of 215°C.
Embodiment 2
[0037] The molar ratio of nitrile, sodium azide and zinc trifluoromethanesulfonate is 1:1:1, the nitrile is benzonitrile, and the water consumption is 10 times of the nitrile mass. 10.3 g (0.1 mol) of benzonitrile, 6.5 g (0.1 mol) of sodium azide, 36.3 g (0.1 mol) of zinc trifluoromethanesulfonate, and the reaction temperature was 90°C.
[0038] Others were the same as in Example 1 to obtain 14.1 g of 5-phenyl-1H-tetrazolium with a yield of 95%, a purity of 98.3%, and a melting point of 215°C.
Embodiment 3
[0040] The molar ratio of nitrile, sodium azide and zinc trifluoromethanesulfonate is 1:1:0.5, the nitrile is benzonitrile, and the water consumption is 30 times of the nitrile mass. 10.3 g (0.1 mol) of benzonitrile, 6.5 g (0.1 mol) of sodium azide, 18.2 g (50 mmol) of zinc trifluoromethanesulfonate, and the reaction time was 6 hours.
[0041] Others were the same as in Example 1 to obtain 13.8 g of 5-phenyl-1H-tetrazolium with a yield of 93%, a purity of 98.5%, and a melting point of 215°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com