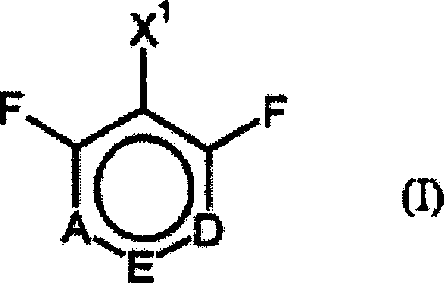
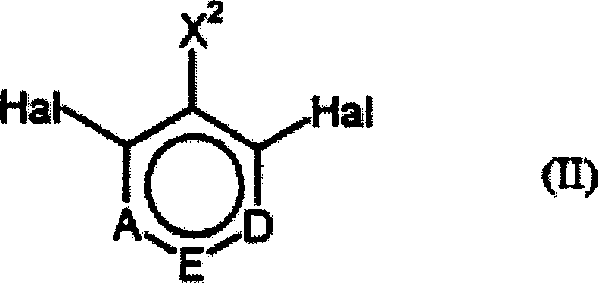
Process for preparing ring-fluorinated aromatics
A technology for compounds and fluorides, which can be used in organic chemistry methods, chemical instruments and methods, preparation of halogenated hydrocarbons, etc., and can solve problems such as low yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0072] The preparation of catalysts of general formula (III) is known and described, for example, in Synthesis 1979, 215-216, Angewandte Chemie 104 , 864, 1992 or EP-A 1 266 904. In some cases it has been observed that in the preparation of compounds of general formula (III) mixtures of two or more individual compounds corresponding to general formula (III) are obtained. These mixtures are also suitable as catalysts for the process of the invention.
[0073] Compared with existing methods, the present invention is used to prepare the method for the aromatic compound of bis or polycyclic-fluorination is to carry out and then finish in one stage, compared with prior art, the method of the present invention is in material, energy source and The tank lining has a low level of technological complexity. Furthermore, the target product is obtained at least equivalent to the prior art, usually in higher yields than the prior art. Therefore, compared with the prior art, the method o...
Embodiment 1
[0081] Embodiment 1: the preparation of 3,5-difluoropyridine
[0082]
[0083] First, 1000 g of diclopyridine and 1580 g of dry potassium chloride were added to 1700 ml of sulfolane in the autoclave. Subsequently, 84 g of CNC catalyst (compound (III-1)) were added, nitrogen was injected to 3 bar, and the mixture was heated to 205° C. while stirring for 48 hours. During the reaction, a maximum total pressure of 12.4 bar was obtained. Next, the mixture was cooled to 10°C and the product was distilled at standard pressure. After further distillation, 473 g of dichloropyridine (60% of theory) were obtained as a colorless liquid.
Embodiment 2
[0084] Example 2 : Preparation of 4,5,6-trifluoropyrimidine
[0085]
[0086] First, 7160ml of sulfolane and 3876g of KF were added to the autoclave, and stirred at 150°C for 1 hour. Subsequently, the mixture was dried by distilling off 700 ml of sulfolane under reduced pressure. The mixture was then cooled to 90°C, flushed with nitrogen, and then pumped into a solution of 3122 g of 4,5,6-trichloropyrimidine and 2386 g of dry sulfolane heated to 45°C. Next, 166g of CNC catalyst and 20.5g of nitrobenzene were added quickly and the vessel was sealed. The mixture was heated to 200°C with stirring for 5 hours, then heated to 220°C for 11 hours. During the reaction, the maximum total pressure was 6.5 bar. The mixture was cooled to 40°C with stirring and slowly depressurized into an ice-cooled receiver. The internal temperature was slowly raised to 150°C and the product was distilled first at standard pressure and then under reduced pressure. After further distillation of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com