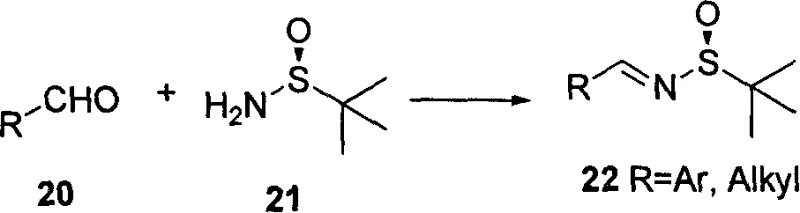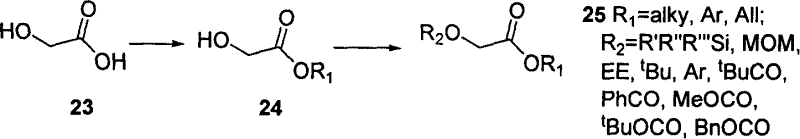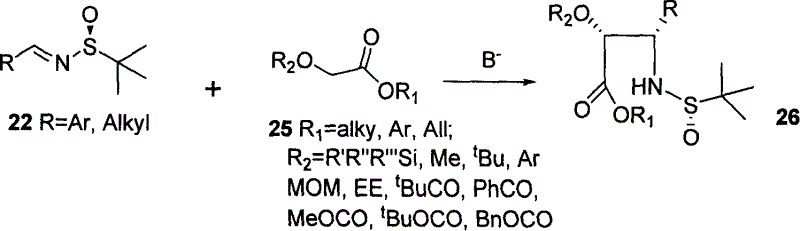Taxol and its analogue side chain synthesizing method
A synthetic method and analogue technology, applied in the field of synthesis of paclitaxel and its analogue side chains, can solve the problems that threaten the long-term persistence and regional distribution of Taxus plants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] (R)-benzaldehyde tert-butyl sulfenamide alkene 22a
[0065]
[0066] Colorless liquid, [α] 20 D -122°(c 1.0, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 ) δ 1.27 (s, 9H), 7.46-7.55 (m, 3H), 7.85-7.88 (m, 2H), 8.60 (s, 1H) ppm.
Embodiment 2
[0068] (R)-p-Chlorobenzaldehyde tert-butyl sulfenamide alkene 22b
[0069]
[0070] White solid, melting point 45-47°C. [α] 20 D -79°(c 2.19, CHCl 3 );. 1 H NMR (400MHz, CDCl 3 )δ1.26(s, 9H), 7.44(d, J=6.4Hz, 2H), 7.78(d, J=6.4Hz, 2H), 8.55(s, 1H); 13 C NMR (CDCl 3 ) delta 22.5, 57.8, 129.2, 130.4, 132.4, 138.5, 161.4 ppm.
Embodiment 3
[0072] (R)-Ortho-bromobenzaldehyde tert-butyl sulfenamide alkene 22c
[0073]
[0074] Colorless liquid, [α] 20 D -211.3° (c 1.23, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 )δ8.94(s, 1H), 8.00(dd, J=7.6, 2.0Hz, 1H), 7.60(dd, J=7.6, 0.8Hz, 1H), 7.28-7.39(m, 2H), 1.24(s , 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com