Vagina medicinal composition
A composition and drug technology, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of high drug cost, large drug dose, and large dose, and achieve improved drug convenience and drug use low dose effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, the preparation of tinidazole vaginal tablet
[0045] prescription:
[0046] Tinidazole 500g
[0047] Lactose 100g
[0048] Starch 50g
[0049] Microcrystalline Cellulose 50g
[0050] Calcium Lactate 30g
[0051] Lactic acid 24g
[0052] 2% HPMC aqueous solution
[0054] Sodium Carboxymethyl Starch 50g
[0055] Make 1000 pieces
[0056] Preparation process: pulverize tinidazole, pass through a 100-mesh sieve, pulverize calcium lactate, pass through a 100-mesh sieve, pass lactose, starch, and microcrystalline cellulose through a 100-mesh sieve respectively, mix the above-mentioned raw and auxiliary materials according to the recipe quantity, and grind them evenly. , pass through a 60-mesh sieve, add lactic acid and an appropriate amount of 2% HPMC aqueous solution to make a soft material, granulate with a 16-mesh sieve, dry at 60°C for 3 hours, add the prescribed amount of magnesium stearate and sodium starch glycol...
Embodiment 2
[0057] Embodiment 2, the investigation of different prescriptions
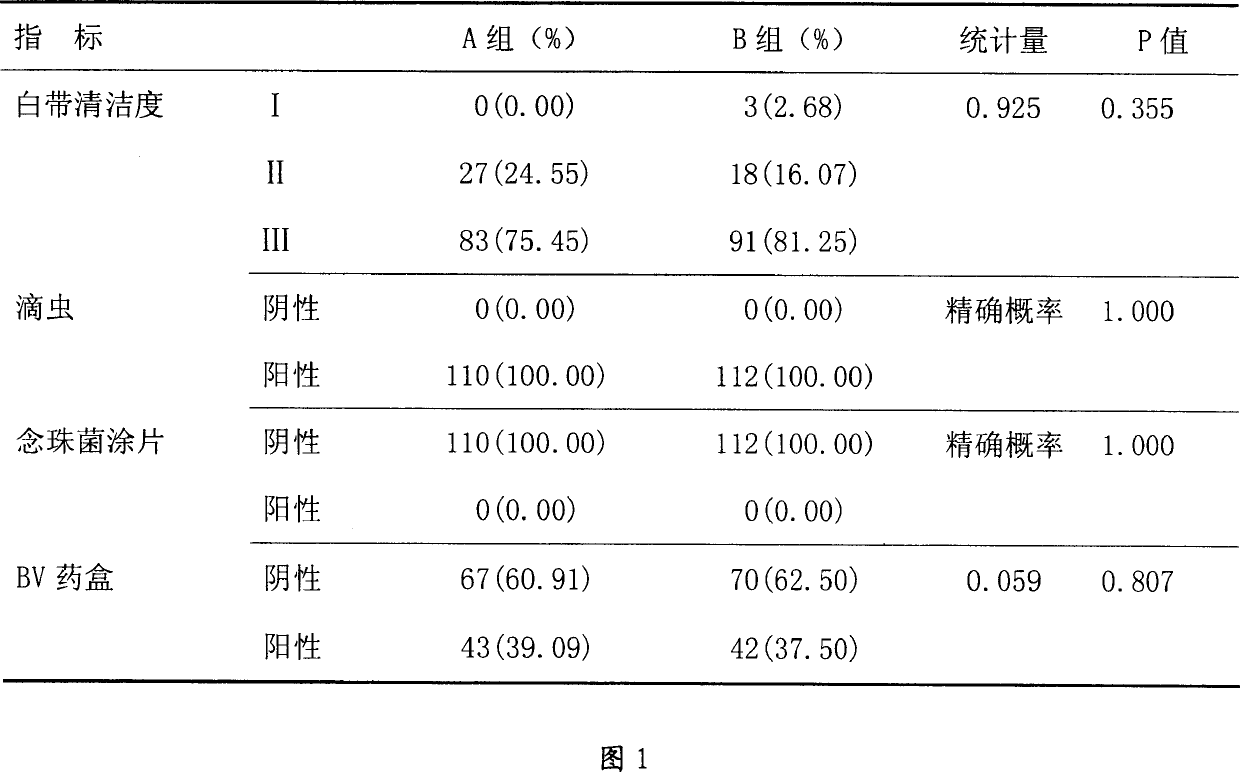
[0058] Different prescriptions are examined to determine the most appropriate range of prescriptions. The inspection methods involved are generally described in the Chinese Pharmacopoeia and classic pharmacy textbooks.
[0059] Prescription I
[0060] Table 2 shows the results of the four formulations examined with hardness, fluidity, melting time, and friability as indicators.
[0061] project
[0062] From the index point of view, the formula II has the best fluidity and hardness after tableting.
Embodiment 3
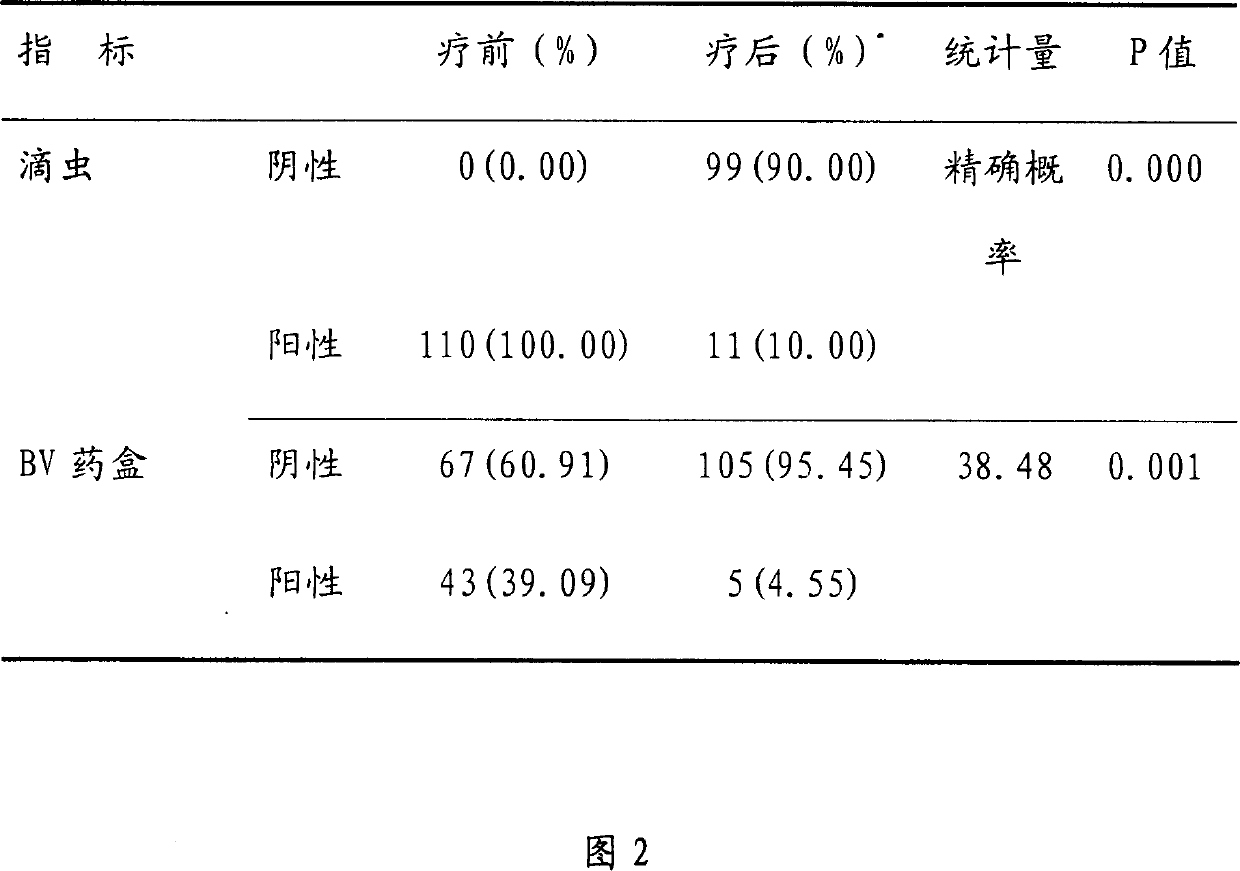
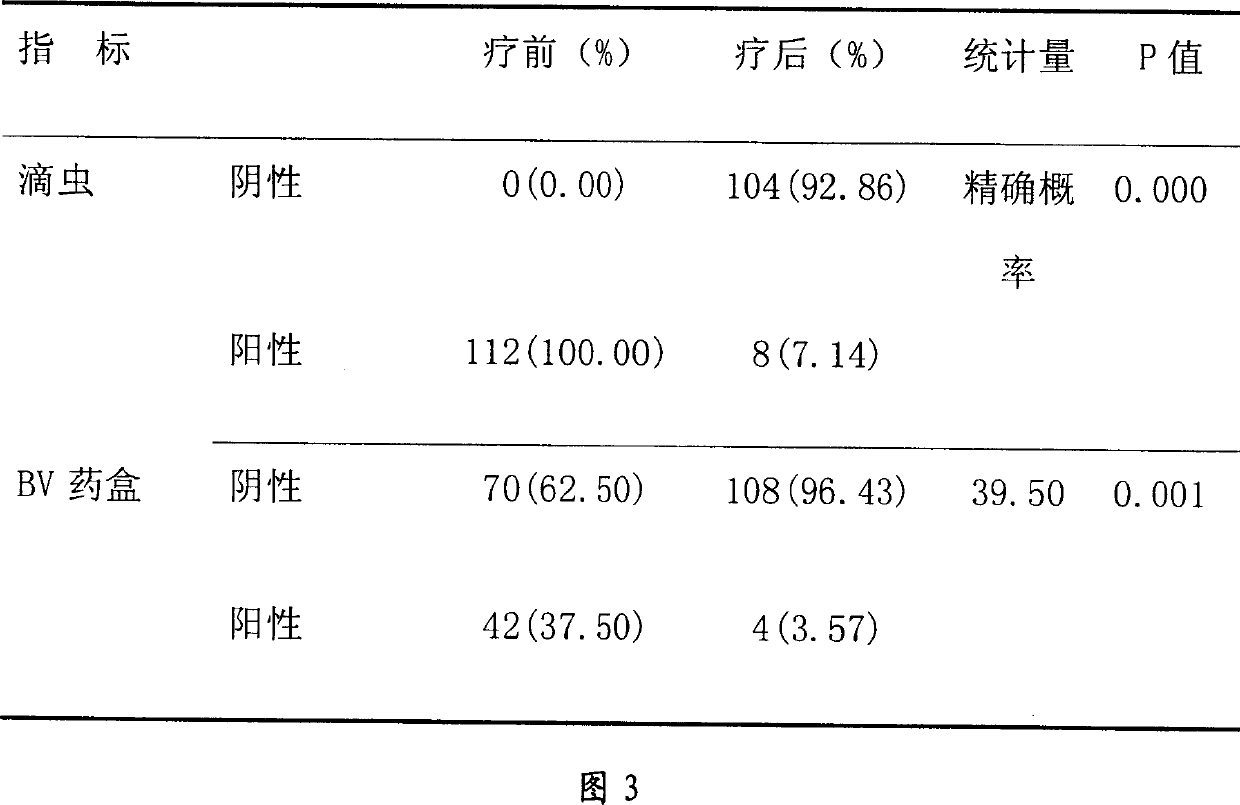
[0063] Embodiment 3, the inspection of lactic acid in tinidazole vaginal tablet
[0064] Because lactic acid is contained in the prescription, the product is further explained by examining the amount of lactic acid added and the pH value of the product.
[0065] Tablets were made according to Example Recipe II by varying the amount of lactic acid. Then grind the tablet finely, get about 2.5g of the product fine powder, add 50ml of water, dissolve, filter, take the filtrate, measure the pH value according to the Chinese Pharmacopoeia 2000 edition two appendix VI (H) method, the results are shown in Table 3.
[0066] Amount of lactic acid (mg / tablet)
[0067] This test found that when the amount of lactic acid was 4-2.0 mg / tablet, there was a better pH range.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com