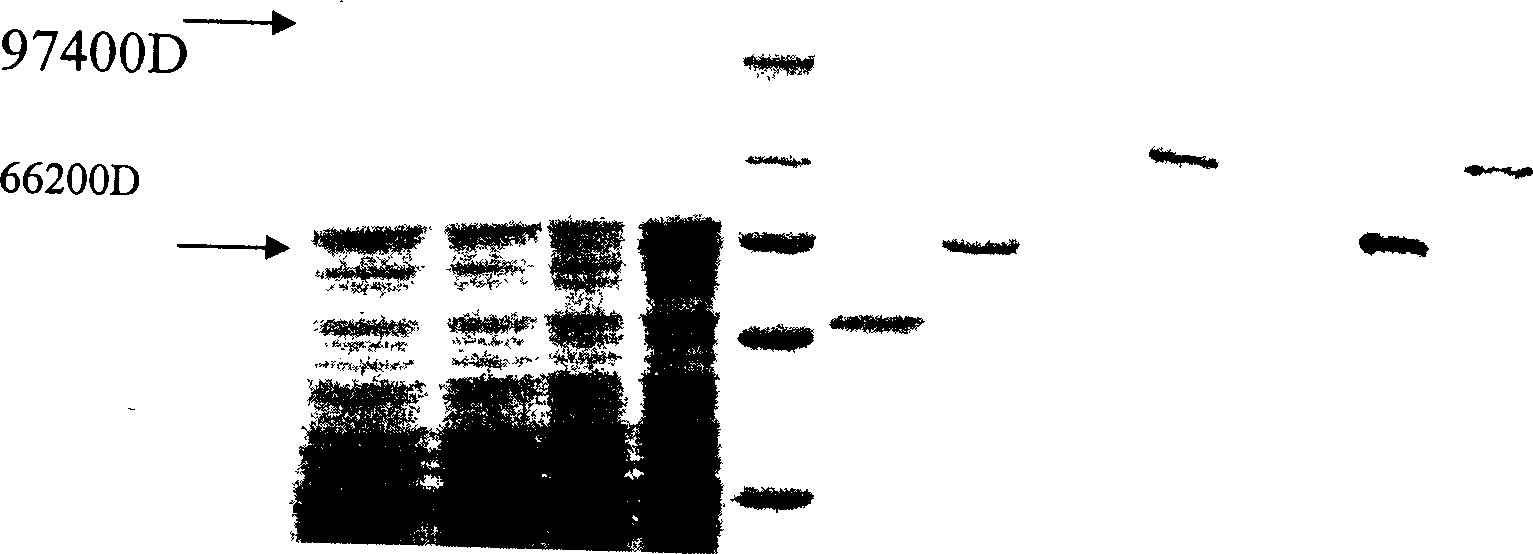Transduction peptide-humanized chloine acetylase fusion protein and its use
A technology of fusion protein and choline acetylation, applied in the direction of transferase, peptide/protein component, fusion polypeptide, etc., can solve the problems of no detection of anti-AchR antibody, decrease of ChAT expression and activity, high technical requirements, etc., and achieve low cost , simplified purification process, high energy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Construction of transduction peptide-CHAT fusion protein carrier
[0032] The s-type of the human ChAT gene donated by Professor Engel of the Mayo Institute of Clinical Neurology in the United States was passed through PCR technology (primer design: the upstream primer is 5'GAATTCACCCTACTCCACAC CAGAGG; the downstream primer is 5'cttaagctaaccctccac. PCR reaction conditions, denaturation at 94°C for 60 sec, 55 ℃ renaturation 60 sec, 72 ℃ extension 90 sec, 30 cycles,) to convert S-ChAT into M-type ChAT.
[0033] according to Figure 5 and Figure 6 The construction route shown is to further construct the eukaryotic expression vector pcDNA-ChAT on the basis of obtaining the cloning vector pGEM-ChAT. pcDNA is a eukaryotic high-efficiency long-term and transient expression vector for mammalian cells. Its promoter CMV enables the vector to be expressed stably and non-replicatively at a high level in most mammalian cells. pGEM-ChAT and pcDNA3.1 (Invitrogen produc...
Embodiment 2
[0036] Example 2 Expression and purification of transduction peptide-CHAT fusion protein
[0037] The recombinant plasmid prepared by the method described in Example 1 pGEX-ChAT Transformed into Escherichia coli BL21 (E.coli BL21(DE3)pLysS was purchased from Beijing Tianwei Times Company), and induced to express the transduction peptide-CHAT fusion protein.
[0038] After culturing the bacteria overnight, re-inoculate 10% of the bacteria in the new LB medium the next day, shake the bacteria to about OD600=0.6, add IPTG with a final concentration of 1.0mmol / L to the medium, and induce culture at 30°C for 4 After 1 hour, the cells were lysed and the GST-transducing peptide-CHAT was extracted, digested with thrombin and purified, and detected by Western blotting.
[0039] Electrophoresis and transmembrane were carried out by conventional methods in Western blotting, goat anti-human CHAT polyclonal antibody (Chemicon International Co.) was added, rabbit anti-goat IgG labeled wit...
Embodiment 3
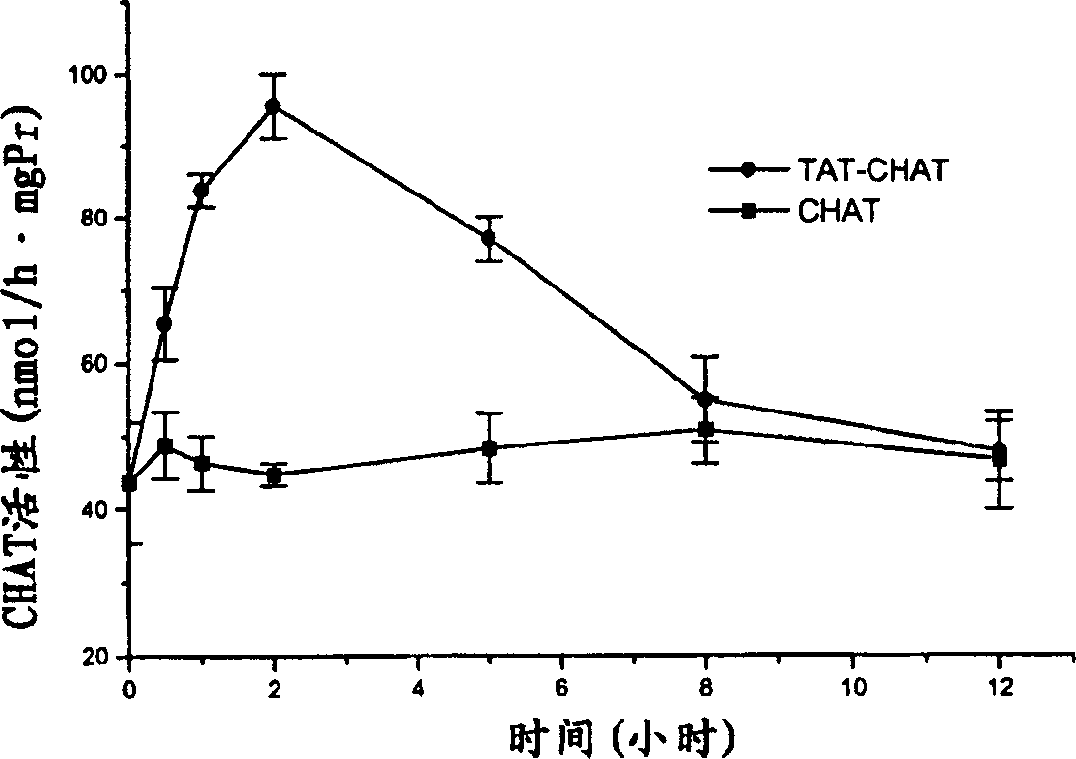
[0041] Example 3 Kinetic Characteristics of CHAT Content in Mouse Brain After Intravenous Injection of Transductive Peptide-CHAT or CHAT
[0042] 1 experimental group
[0043] Healthy male mice (Shanghai species, class II animals), body weight 25±1g. Provided by Animal Experiment Center of Academy of Military Medical Sciences. The mice were randomly divided into 2 groups with 3 mice in each group. The mice in group 1 were injected with 100 μg transducible peptide-CHAT or CHAT through the tail vein, and the brains were removed at different time points (0, 0.5, 1, 2, 5, 12 h) after the injection, and the CHAT activity was measured. The brains of mice in group 2 were injected with different concentrations (0-500 μg / mL) of transduction peptide-CHAT or CHAT 0.2 ml into their tail veins 2 hours later, and the CHAT activity was measured. Each of the above experiments was repeated 3 times.
[0044] 2 Determination of CHAT activity
[0045] Animals in each group were killed by dec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com