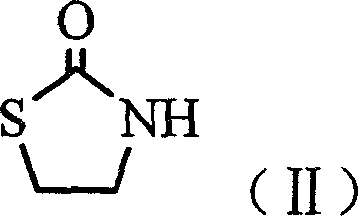
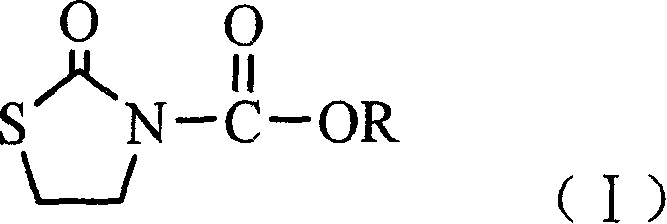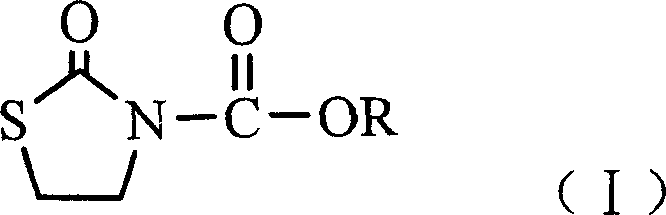N-alkoxide oxo-2-triazolidone derivative, its preparation and use
A technology of thiazolidinone and alkoxycarbonyl, applied in the field of N-alkoxycarbonyl-2-thiazolidinone derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Synthesis of N-pentyloxycarbonyl-2-thiazolidinone
[0024] Add 19.8g (0.067mol) of bis(trichloromethyl)carbonate and 50ml of dichloromethane into a 250ml three-necked flask, and slowly add n-amyl alcohol (0.2mol) under stirring. Under cooling in an ice bath, a solution of triethylamine (0.2 mol) and 10 ml of dichloromethane was slowly added dropwise, maintaining the temperature between 0 and 5°C. After the dropwise addition was completed, the temperature was raised slowly and stirred at 20° C. for 2 hours. Wash with water three times (3×50ml), dry over anhydrous sodium sulfate, filter, concentrate, and distill out n-pentyl chloroformate under reduced pressure. Yield 85.6%.
[0025] 2-Thiazolidinone (5mmol) and triethylamine (0.72g, 7mmol) were dissolved in 10ml of dichloromethane and stirred. Under cooling in an ice bath, the above n-amyl chloroformate (6 mmol) was added dropwise. After the dropwise addition was completed, the reaction was carried out at 0...
Embodiment 2
[0033] Example 2 Synthesis of N-isoamyloxycarbonyl-2-thiazolidinone
[0034] Add 19.8 g (0.067 mol) of bis(trichloromethyl) carbonate and 50 ml of chloroform into a 250 ml three-neck flask, and slowly add isoamyl alcohol (0.2 mol) while stirring. Under cooling in an ice bath, a solution of pyridine (0.2 mol) and 10 ml of chloroform was slowly added dropwise, maintaining the temperature between 0 and 5°C. After the dropwise addition was completed, the temperature was raised slowly and stirred at 25° C. for 2 hours. Wash with water three times (3×50ml), dry over anhydrous sodium sulfate, filter, concentrate, and evaporate isoamyl chloroformate under reduced pressure. Yield 82.7%.
[0035] 2-Thiazolidinone (5mmol) and triethylamine (0.82g, 8mmol) were dissolved in 15ml of dichloromethane and stirred. Under cooling in an ice bath, the above-mentioned isoamyl chloroformate (6 mmol) was added dropwise. After the dropwise addition was completed, the reaction was carried out at 0-...
Embodiment 3
[0043] Example 3 Synthesis of N-butoxycarbonyl-2-thiazolidinone
[0044] N-pentanol is replaced by n-butanol, and others are the same as in Example 1 to obtain n-butyl chloroformate. Yield 88.3%.
[0045] 2-Thiazolidinone (5mmol) and pyridine (0.56g, 7mmol) were dissolved in 10ml of chloroform and stirred. Under cooling in an ice bath, the above n-butyl chloroformate (6 mmol) was added dropwise. After the dropwise addition was completed, the reaction was carried out at 0-10°C for 9 hours. Washed three times with water, dried over anhydrous sodium sulfate, filtered and concentrated. The residue was separated and purified by column chromatography [V (petroleum ether): V (ethyl acetate) = 3: 1] to obtain 0.77 g of light yellow transparent oily N-n-butoxycarbonyl-2-thiazolidinone (compound No. 6 ). The yield was 76.3%.
[0046] Elemental Analysis Values:
[0047] C H N
[0048] Measured value (%): 47.43 6.69 6.85
[0049] Calculated value (%): 47.27 6.44 6.89
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com