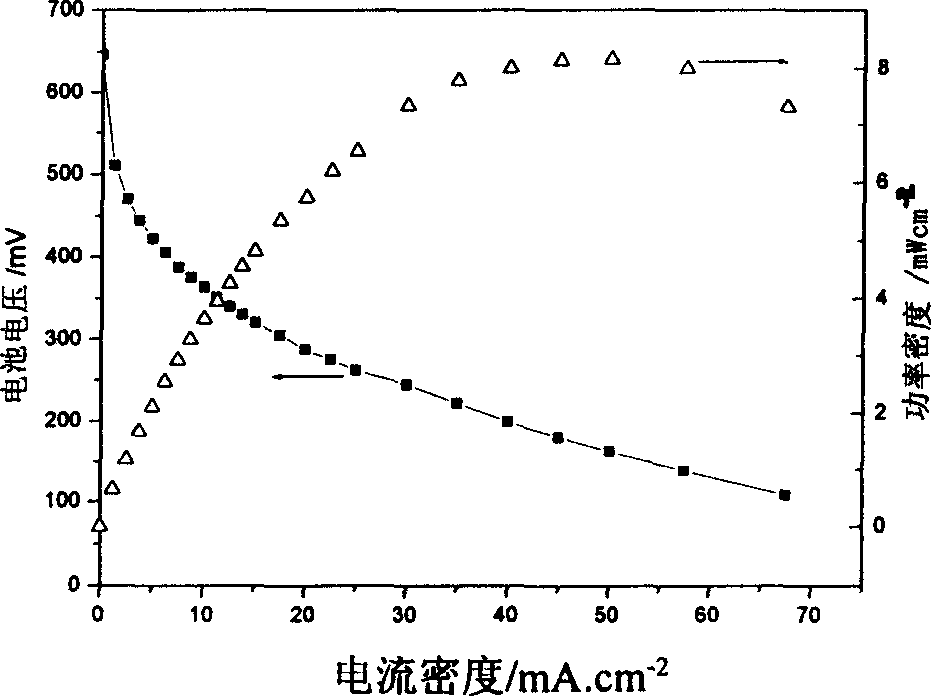
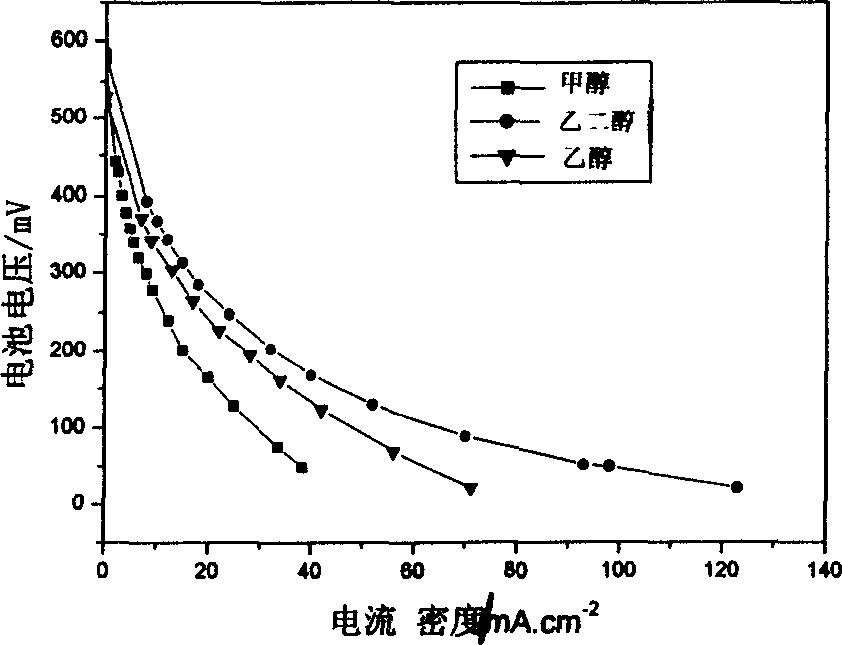
Use of polymerized phthalocyanine compound as cell cathode catalyst with direct alcohols as fuel
A technology of compound and phthalocyanine, which is applied in the application field of polymerizing phthalocyanine compound as the cathode catalyst of direct alcohol fuel cell, which can solve the problems of phthalocyanine ring damage, low catalyst activity and insufficient stability, etc. The effect of insufficient resources and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 n=0; X=N, R=-O 2 ; M=Fe Synthesis of six (4-nitro) bicyclic phthalocyanine iron (II) [HNODPcFe (II)]
[0038] Add 150mL of refined kerosene, 15.6g of 4-nitrophthalimide, 16g of urea and 2.95g of pyromellitic anhydride into a three-necked flask equipped with a stirrer, a reflux condenser and a thermometer, and heat to 140°C , Stir for 1 hour until the mixture is uniform, and keep at 180°C for 4 hours. After cooling to about 80°C, add 16g of urea, 0.4g of ammonium molybdate and 10.6g of ferrous ammonium sulfate, and stir for 0.5h. The reaction was stirred at 180°C for 3h. Cool to 80°C and add 5g of urea, stir and react at 180-220°C for 3h. Obtained black pellets. Suction was crude product.
[0039] The purification of the sample adopts the method of dissolving in concentrated sulfuric acid and diluting with water, the yield is 53.8%
[0040] Its NMR data are: 6.95~7.20 (multiple peaks), 8.07~8.10 (double peaks), 8.45~8.62 (triplet peaks), 8.94 (single peak...
Embodiment 2
[0042] In Example 1, the complex hexa(4-nitro)bicyclic phthalocyanine cobalt(II) [HNODPcCo(II)] in which the central metal ion is a cobalt ion can be obtained by using cobalt chloride instead of ferrous ammonium sulfate.
[0043] If other salts are used instead of ferrous ammonium sulfate, complexes of different metal ions can be obtained.
Embodiment 3
[0045] In Example 1, if the molar ratio of 4-nitrophthalimide and pyromellitic anhydride is controlled to be 4:1, a polymeric phthalocyanine complex with n=1 can be prepared. The molar ratio is 10:3, and the polymerized phthalocyanine complex with n=2 can be obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com