Catalytic hydrolysis method of circulation phase transition for preparing benzaldehyde and benzaldehyde containing substituent
A technology of phase transfer catalysis and phase transfer catalyst, which is applied in the hydrolysis to prepare carbonyl compounds, organic chemistry, etc., can solve the problems of environmental heavy metal pollution, low reactor volume utilization rate, small reactor volume utilization rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
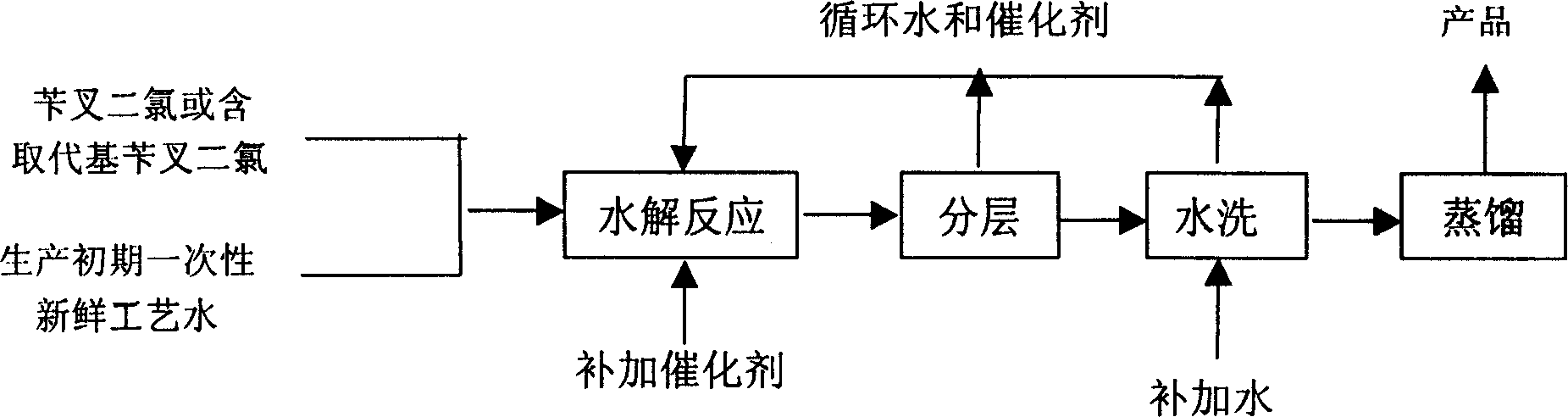
[0016] In the 500ml flat-bottomed three-neck reactor with magnetic stirring, add 50g benzylidene dichloride (self-made, purity 99%), the water that weight is m1 and the benzyltriethylammonium chloride that weight is m2 respectively, control certain The reaction temperature is T1, and the reaction is stirred for t1 minutes until the reaction of the benzylidene dichloride is completed. The reaction solution was left to stand and separated to obtain an organic phase and an aqueous phase, respectively. The organic phase is weighed to obtain the weight m3, and the area percentage is determined by gas chromatography, and the mass percentage content x of benzaldehyde is calculated. See Table 1 for test results.
[0017] No.
[0018] * In Experiments 6-9, the aqueous phase of the reaction solution from Experiment 2 was used for recycling, without adding water and catalyst.
Embodiment 2
[0020] In the 500ml flat-bottomed three-neck reactor with magnetic stirring, add 50g p-chlorobenzylidene dichloride respectively (self-made, purity 99%), the water that weight is m1 and the tetraethylammonium chloride that weight is m2, control certain The reaction temperature is T1, and the reaction is stirred for t1 minute until the p-chlorobenzylidene dichloride is completely reacted. The reaction solution was kept warm and allowed to stand for stratification to obtain an organic phase and an aqueous phase respectively. The organic phase is weighed to obtain the weight m3, and the area percentage is determined by gas chromatography, and the mass percentage content x of p-chlorobenzaldehyde is calculated. See Table 2 for test results.
[0021] No.
[0022] * In Experiments 7-9, the aqueous phase of the reaction solution from Experiment 5 was used for recycling, without adding water and catalyst.
Embodiment 3
[0024] In the 500ml flat-bottomed three-neck reactor with magnetic stirring, add 50g o-chlorobenzylidene dichloride respectively (self-made, purity 99%), the water that weight is m1 and the tetrabutylammonium chloride that weight is m2, control certain The reaction temperature is T1, and the reaction is stirred for t1 minutes until the reaction of o-chlorobenzylidene dichloride is completed. The reaction solution was left to stand and separated to obtain an organic phase and an aqueous phase, respectively. The organic phase is weighed to obtain the weight m3, and the area percentage is determined by gas chromatography, and the mass percentage content x of o-chlorobenzaldehyde is calculated. See Table 3 for test results.
[0025] No.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com