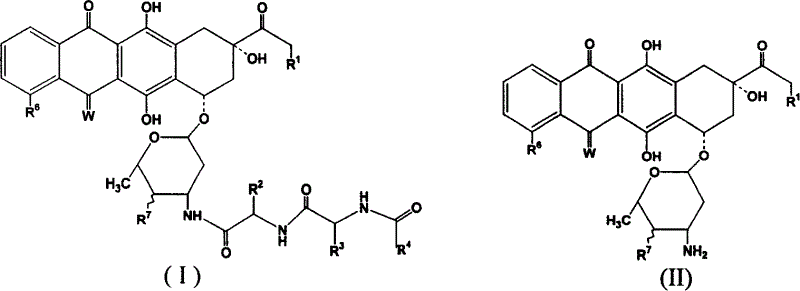
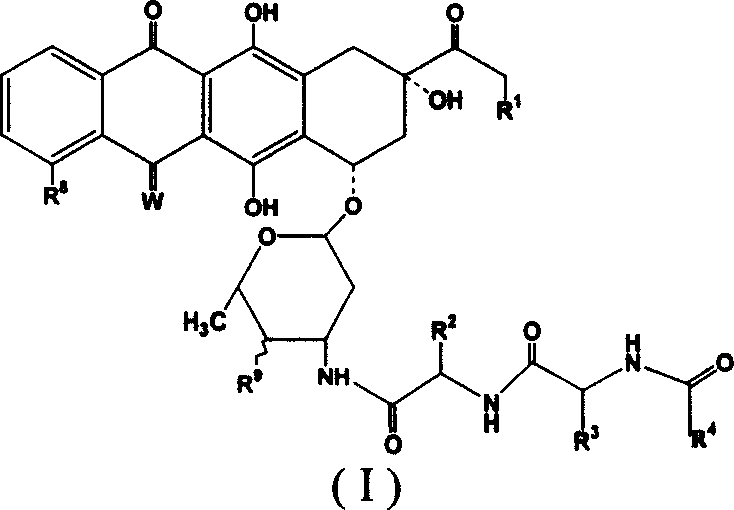
Adriacin derivative with anti-cancer activity
A drug active and neutral technology, applied in the field of doxorubicin derivatives with anticancer activity, can solve the problems of high cost and complex production process, and achieve high social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1, the preparation of stearoyl-L-alanine-L-proline-doxorubicin (compound one)
[0054] Dissolve stearyl dipeptide (50mg, 110nmol) and N-hydroxysuccinimide (13mg, 110nmol) in 15ml of ethylene glycol dimethyl ether, cool the solution to 0-5°C, and add DCC (23mg, 120nmol), and then reacted at room temperature for 5h. Adriamycin hydrochloride (58 mg, 100 nmol), 0.2 ml of triethylamine, and 2 ml of DMF were added to the reaction system. React overnight at room temperature. Ethylene glycol dimethyl ether was removed by subtractive evaporation, 50ml of ethyl acetate was added to the residue, cyclohexylurea was removed by filtration, the ethyl acetate solution was washed once with 1N aqueous hydrochloric acid solution, once with saturated aqueous sodium chloride solution, and the aqueous layers were combined and washed with Back-extract once with 30 ml of ethyl acetate, combine the ethyl acetate layers, and dry over anhydrous sodium sulfate. Filter and evaporate t...
Embodiment 2
[0055] Embodiment 2, the preparation of oleoyl-L-alanine-L-proline-doxorubicin (compound 2)
[0056] Using the preparation method of Example 1, it is prepared by replacing stearoyl-L-alanine-L-proline with oleoyl-L-alanine-L-proline. 1 H NMR (CDCl 3 , ppm) δ13.94(s, 1H), 13.22(s, 1H), 8.02(d, 1H, J=7.6Hz), 7.77(dd, 1H, J=7.6, 8.4Hz), 7.37(d, 1H , J=8.4Hz), 6.94(d, 1H, J=7.2Hz), 6.62(d, 1H, J=8.0Hz), 6.33(brs, 1H), 5.48(s, 1H), 5.24-5.42(m , 2H), 5.24(s, 1H), 4.76(s, 2H), 4.62(brs, 1H), 4.40-4.50(m, 1H), 4.10-4.18(m, 3H), 4.05(s, 3H), 3.66(s, 1H), 3.26(d, 1H, J=18.8Hz), 3.00(d, 1H, J=18.8Hz), 2.75(d, 1H, J=6.4Hz), 2.74(d, 1H, J =6.4Hz), 2.33(d, 1H, J=14.48Hz), 2.10-2.23(m, 6H), 1.75-1.92(m, 2H), 1.52-1.62(m, 2H), 1.44(d, 3H, J=7.2Hz), 1.20-1.40 (m, 23H), 0.80-0.95 (m, 9H).
Embodiment 3
[0057] Embodiment three, the preparation of linoleoyl-L-alanine-L-proline-doxorubicin (compound three)
[0058] Using the preparation method of Example 1, it is prepared by replacing stearoyl-L-alanine-L-proline with linoleoyl-L-alanine-L-proline. 1 H NMR (CDCl 3 , ppm) δ13.95(s, 1H), 13.23(s, 1H), 8.02(d, 1H, J=7.6Hz), 7.77(dd, 1H, J=7.6, 8.4Hz), 7.37(d, 1H , J=8.4Hz), 6.94(d, 1H, J=7.2Hz), 6.62(d, 1H, J=8.0Hz), 6.33(brs, 1H), 5.48(s, 1H), 5.24-5.42(m , 4H), 5.24(s, 1H), 4.76(s, 2H), 4.62(brs, 1H), 4.40-4.50(m, 1H), 4.10-4.18(m, 3H), 4.05(s, 3H), 3.66(s, 1H), 3.26(d, 1H, J=18.8Hz), 3.00(d, 1H, J=18.8Hz), 2.75(d, 1H, J=6.4Hz), 2.74(d, 1H, J =6.4Hz), 2.33(d, 1H, J=14.48Hz), 2.10-2.23(m, 6H), 1.75-1.92(m, 2H), 1.52-1.62(m, 2H), 1.20-1.40(m, 22H), 0.80-0.95 (m, 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com