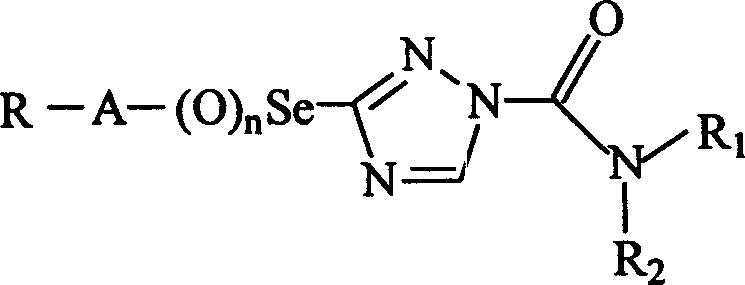
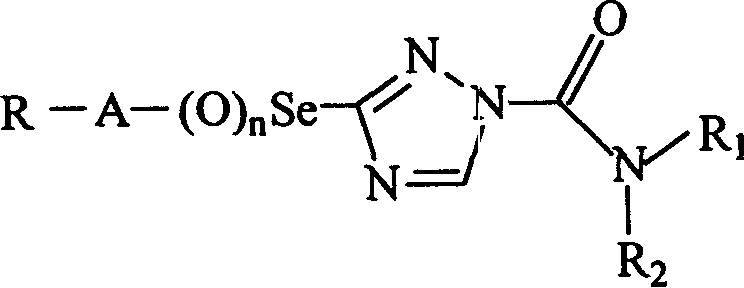Cesium triazoleamide compounds and their preparation and use
A technology of selenium triazole amide and compound, which is applied in the field of selenium triazole amide compound and its preparation and application, can solve the problems of crop phytotoxicity, and achieve the effect of improving safety and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
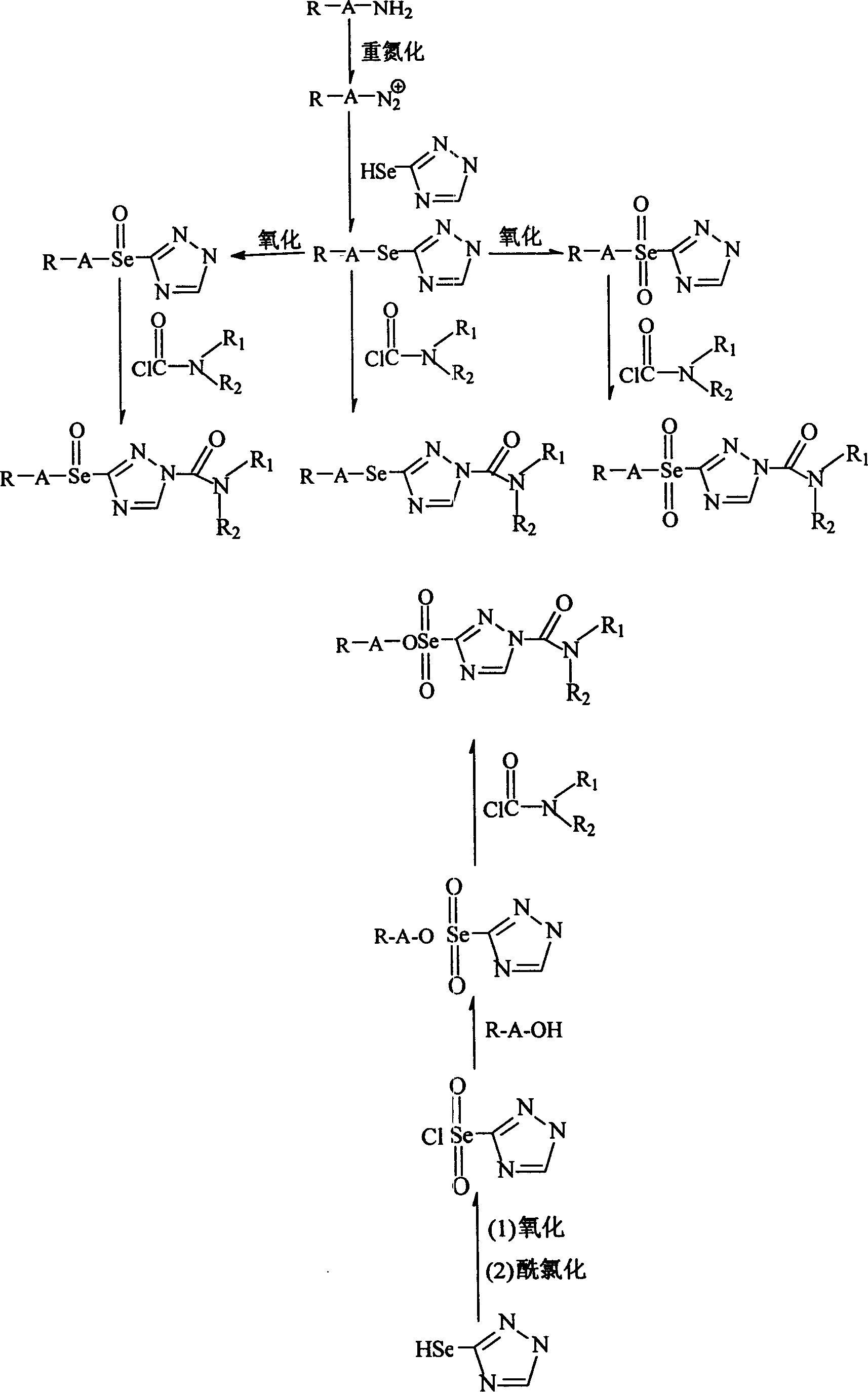
[0030] Synthesis of 1-(N,N'-diethylamino)formyl-3-(1H-1,2,4-triazole)selenoyl-2,4,6-trimethylbenzene.
[0031] (1) Synthesis of seleno-semicarbazide:
[0032] Add 24.5g (0.27mol) of crushed thiosemicarbazide, 150ml of absolute ethanol and 16.8ml (0.27mol) of methyl iodide into a 250ml single-necked flask, and react under reflux for one hour. A transparent colorless ethanol solution of S-methylthiosemicarbazide was obtained. Under an ice bath, 15 g (0.405 mol) of sodium borohydride and 21.32 g (0.27 mol) of selenium powder were added to a 500 ml three-necked flask and mixed uniformly. After vacuumizing the system and argon, inject 150ml of absolute ethanol to react violently. After the reaction is over, go to the ice bath. Inject the prepared ethanol solution of S-methylthiosemicarbazide into the system, and react overnight at room temperature. Filter, wash with absolute ethanol, and dry to obtain off-white selenosesemicarbazide with a yield of 90%. Recrystallize from abso...
Embodiment 2
[0044] Synthesis of 1-(N,N'-diethylamino)formyl-3-(1H-1,2,4-triazole)selenoyl-(2,4,6-trimethylbenzene)
[0045] (1) Synthesis of triazolyl selenic acid:
[0046] In a 100ml one-necked flask, add 2.96g (0.02mol) of triazolylselenophene and 25ml of glacial acetic acid to dissolve. Slowly add 20ml of 30% hydrogen peroxide dropwise, and react overnight at room temperature. Filter, wash with cold water and dry to give a white solid. Yield 61%, M.p. 52°C.
[0047] (2) Synthesis of triazolyl selenoyl chloride:
[0048] In a 50ml one-necked flask, add 1.96g (0.01mol) of triazolyl selenic acid and 25ml of thionyl chloride, and react overnight at 40-45°C. Colorless liquid distilled under reduced pressure. Yield 85%.
[0049] (3) Synthesis of triazole selenoyl ester group-(2,4,6-trimethylbenzene):
[0050] 0.68 g (0.005 mol) of 2,4,6-trimethylphenylphenol and 25 ml of chloroform were added to a 50 ml one-necked flask to dissolve, and 0.98 g (0.005 mol) of triazolyl selenoyl chloride...
Embodiment 3
[0054] Synthesis of 1-(N,N'-diethylamino)formyl-3-(1H-1,2,4-triazole)selenoyl-(2,4,6-trimethylbenzene)
[0055] (1) Synthesis of 1H-1,2,4-triazoleselenoyl-(2,4,6-trimethylbenzene):
[0056] Add (2,4,6-trimethylphenyl)selenoether-1H-1,2,4-triazole (refer to example 1) 1.34g (0.005mol) and 30ml of glacial acetic acid in a 100ml single-necked flask, drop slowly Add 10ml of 30% hydrogen peroxide. React at room temperature for 10 hr, extract with dichloromethane, concentrate the extract and separate on a silica gel column (methanol:ethyl acetate=1:8) to obtain a white solid. Yield 78%, M.p. 202-204°C.
[0057] (2) 1-(N,N'-diethylamino)formyl-3-(1H-1,2,4-triazole)selenoyl-(2,4,6-trimethylbenzene) synthesis:
[0058] Using the (2,4,6-trimethylphenyl)selenoyl-1H-1,2,4-triazole synthesized above as a raw material, a white solid was obtained according to the method (4) in Example 2. Yield 87%, M.p. 95-96°C. MS (ESI) m / z: 405 (M+Na + ), element analysis: theoretical value C 50.40%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com