Chitose microsphere and microcapsule with uniform size and its preparation method
A technology of chitosan microspheres and chitosan, which is applied in the field of pharmaceutical preparations in medical engineering, can solve the problems of poor dispersion, uneven particle size of chitosan drug carriers, and low embedding rate, and achieve uniform particle size, Maintain high activity and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
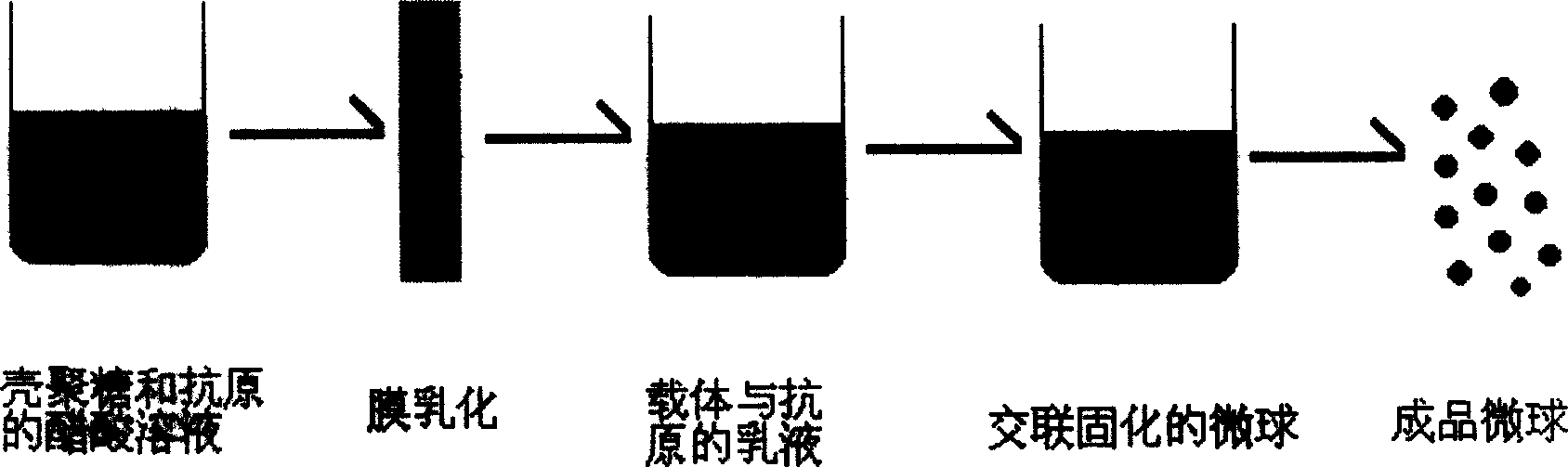
[0037] The hydrophobic membrane with a pore size of 4.7 μm is immersed in a lipophilic substance to fully wet the porous membrane to ensure that the hydrophobic chains on the membrane are completely stretched. Prepare 1wt% acetic acid aqueous solution, add a certain amount of NaCl and dissolve it under stirring, its concentration is 1wt%, then add a certain amount of chitosan to make its concentration 2wt%, after it is completely dissolved, filter the solution spare. Add the oil-soluble emulsifier to 60 ml of the mixed oil phase of liquid paraffin and petroleum ether, and stir until completely dissolved to form the oil phase. The water phase of 6.0g is pressed into the oil phase through a hydrophobic microporous membrane with a uniform pore size under a certain pressure to obtain a W / O type emulsion; GST is slowly added dropwise in the resulting emulsion for cross-linking (amino and aldehyde groups) The molar ratio is 1: 1), and the crosslinking is carried out at normal tempe...
Embodiment 2
[0041] The hydrophobic membranes with pore diameters of 4.7 μm, 5.7 μm, 13 μm, and 19.6 μm were soaked in lipophilic substances, so that the porous membranes were fully wet to ensure that the hydrophobic chains on the membranes were completely stretched. Prepare 1wt% acetic acid aqueous solution, add a certain amount of NaCl and make it dissolve under stirring, its concentration is 1wt%, then add a certain amount of chitosan to make its concentration 1.5wt%, after it is completely dissolved, the solution Filter and set aside. Add the oil-soluble emulsifier to 60 ml of the mixed oil phase of liquid paraffin and petroleum ether, and stir until completely dissolved to form the oil phase. 6.0g of the water phase was pressed into the oil phase through various hydrophobic microporous membranes with uniform pore size under a certain pressure to obtain a W / O emulsion; in the obtained emulsion, GST was slowly added dropwise for cross-linking (amino and The mol ratio of aldehyde group ...
Embodiment 3
[0043] The hydrophobic membrane with a pore size of 4.7 μm is immersed in a lipophilic substance to fully wet the porous membrane to ensure that the hydrophobic chains on the membrane are completely stretched. Prepare 1wt% acetic acid aqueous solution, add a certain amount of NaCl and make it dissolve under stirring, its concentration is 1wt%, then add a certain amount of chitosan to make its concentration respectively 1.0wt%, 1.5wt% and 2.0wt% , after it was completely dissolved, the solution was filtered for later use. Add the oil-soluble emulsifier to 60 ml of the mixed oil phase of liquid paraffin and petroleum ether, and stir until completely dissolved to form the oil phase. The water phase of 6.0g is pressed into the oil phase through a hydrophobic microporous membrane with a uniform pore size under a certain pressure to obtain a W / O type emulsion; GST is slowly added dropwise in the resulting emulsion for cross-linking (amino and aldehyde groups) The molar ratio is 1: ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com