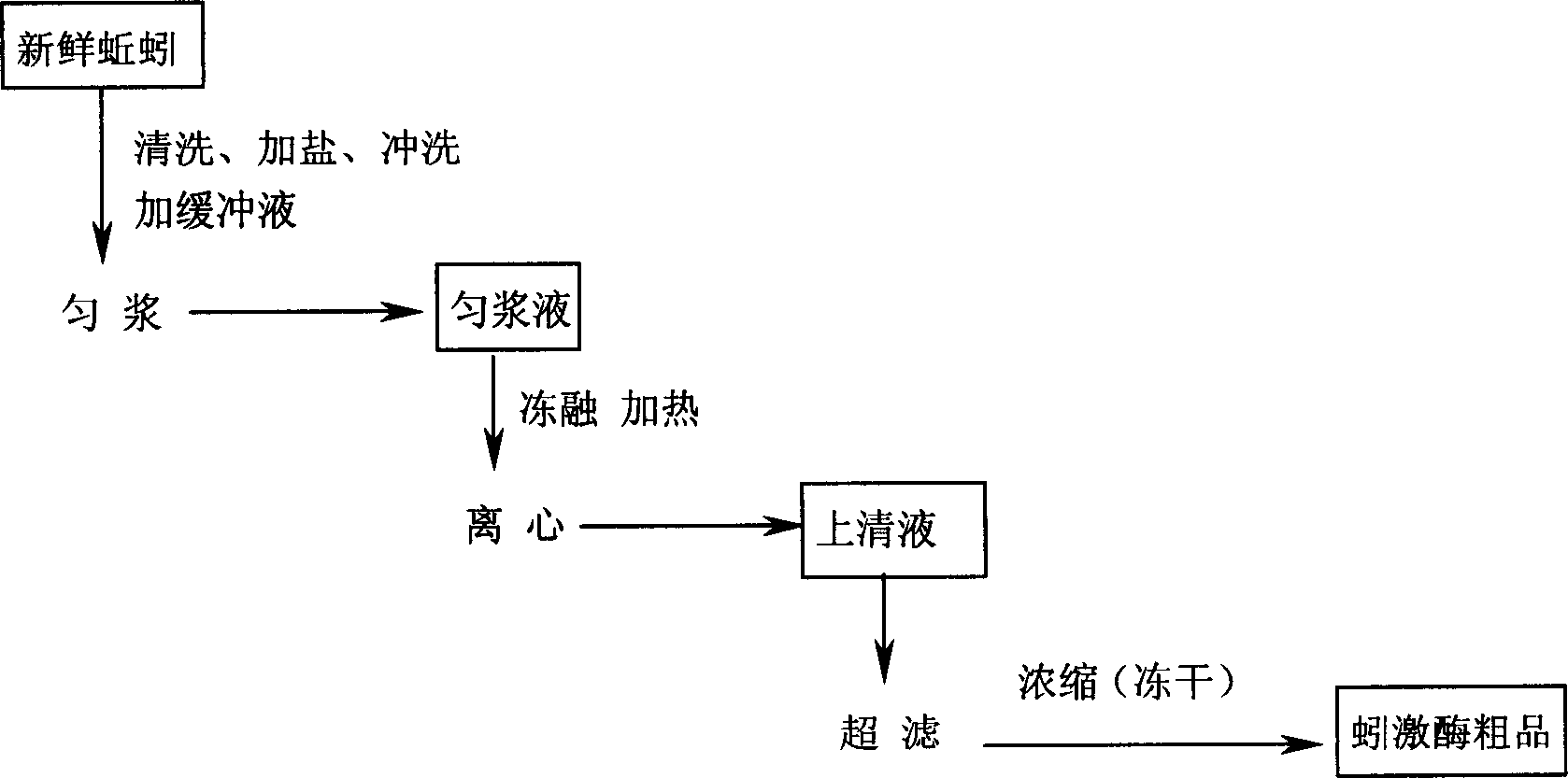
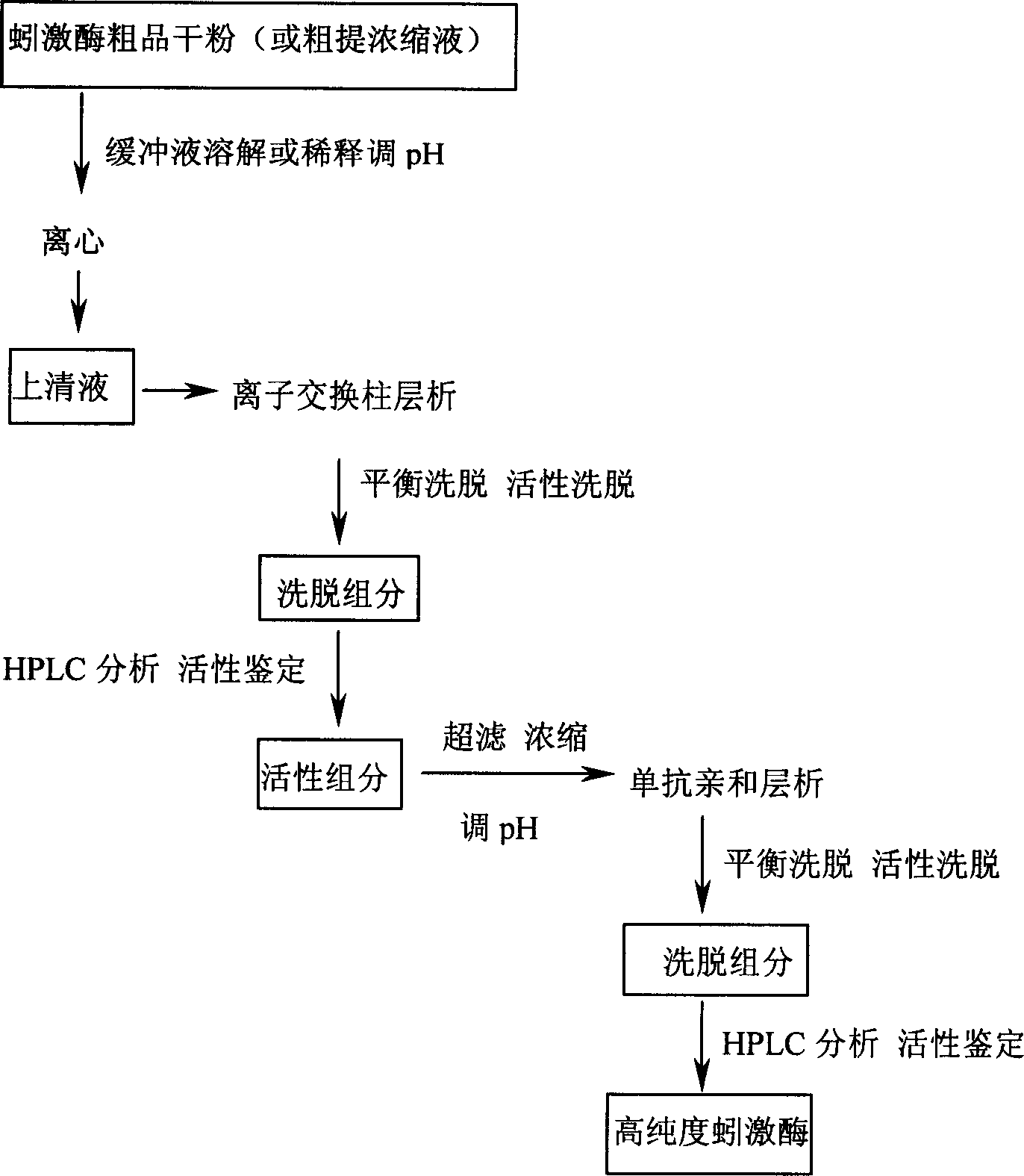
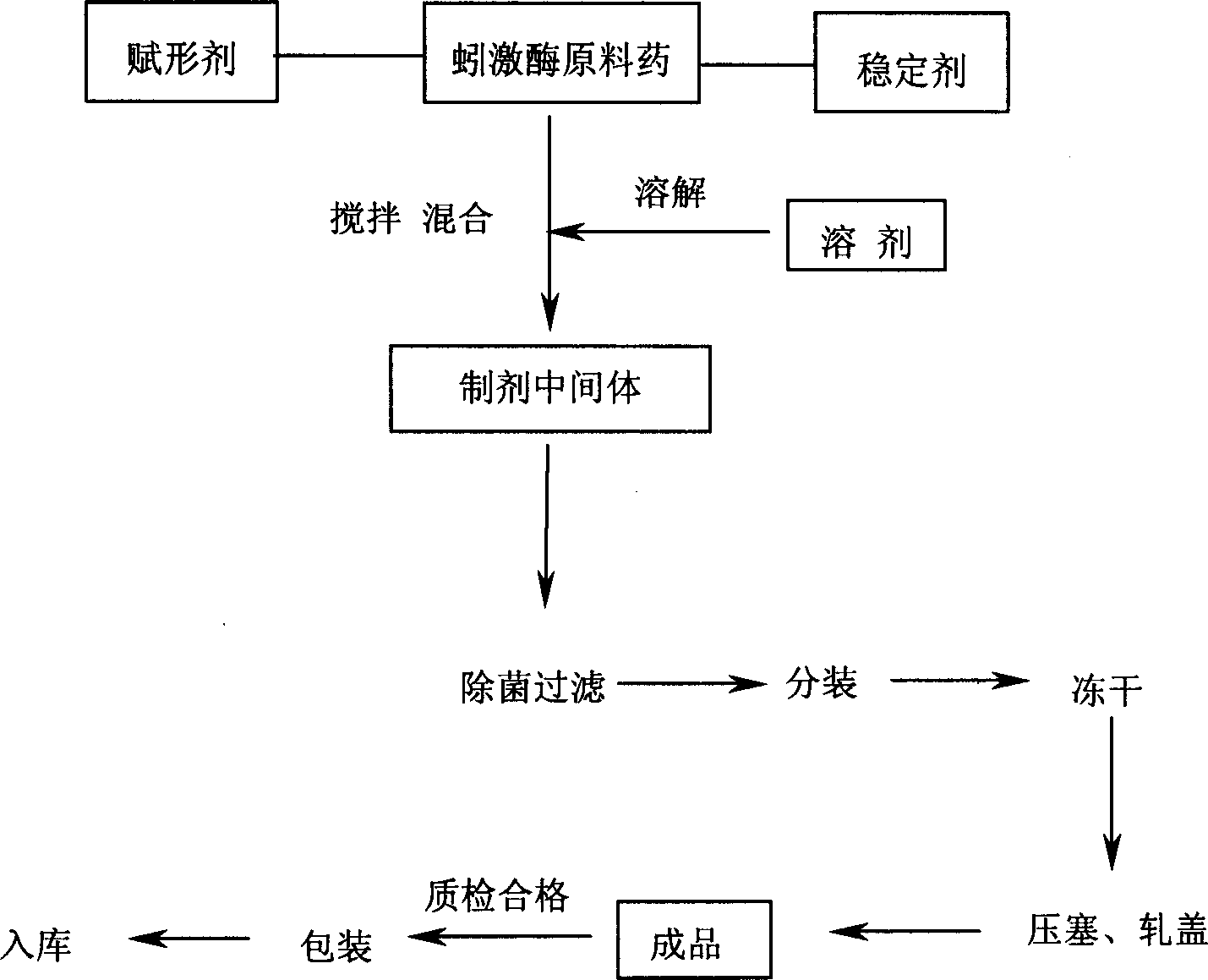
Preparation method for high purity lumbrukinase and pharmaceutical preparation made therefrom
A lumbrokinase, high-purity technology, applied in the field of preparation of high-purity lumbrokinase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Lumbrokinase 50 μg / ml purified by high performance liquid chromatography and mixed with an equal volume of complete adjuvant and emulsified as a stimulus, immunized 7-week-old BALB / c mice subcutaneously, 0.2ml each time, once a week , a total of 6 times of immunization. Three days before the fusion, 0.2ml stimulator mice were injected into the tail vein for booster immunization.
[0043] Take the immunized mouse splenic B lymphocyte suspension and mix it with mouse sp2 / 10 myeloma cells at a ratio of 5:1, add 50% polyethylene glycol with a molecular weight of 40000, and inoculate the cell mixture in multiple 96 wells in the wells of the cell culture plate.
[0044] Place the cell culture plate inoculated with the cell mixture in CO 2 Cultured in an incubator. The HAT medium containing 15% fetal bovine serum was used for the 1st to 4th day, the HT medium was used for the 5th to 10th day, and the RPM1-1640 medium containing 10% fetal bovine serum was used for cultivatio...
Embodiment 2
[0047] Lumbrokinase 250 μg / ml purified by high-performance liquid chromatography and emulsified with an equal volume of complete adjuvant was mixed and emulsified as a stimulus, and immunized by subcutaneous injection to 7-week-old BALB / c mice, 0.2ml each time, once a week , a total of 6 times of immunization. Three days before the fusion, 0.2ml stimulator mice were injected into the tail vein for booster immunization.
[0048] Take the immunized mouse splenic B lymphocyte suspension and mix the mouse sp2 / 10 myeloma cells at a ratio of 8:1, add 50% polyethylene glycol with a molecular weight of 50000, and inoculate the cell mixture in multiple 96 wells in the wells of the cell culture plate.
[0049] Place the cell culture plate inoculated with the cell mixture in CO 2 Cultured in an incubator. The HAT medium containing 15% fetal bovine serum was used for the 1st to 4th day, the HT medium was used for the 5th to 10th day, and the RPM1-1640 medium containing 10% fetal bovine...
Embodiment 3
[0052] Lumbrokinase 500μg / ml purified by high performance liquid chromatography and emulsified with an equal volume of complete adjuvant was emulsified as a stimulus, and immunized by subcutaneous injection of 0.2ml each time to 7-week-old BALB / c mice, once a week , a total of 6 times of immunization. Three days before the fusion, 0.2ml stimulator mice were injected into the tail vein for booster immunization.
[0053] Take the immunized mouse splenic B lymphocyte suspension and mix it with mouse sp2 / 10 myeloma cells at a ratio of 10:1, add 50% polyethylene glycol with a molecular weight of 60000, and inoculate the cell mixture in multiple 96 wells in the wells of the cell culture plate.
[0054] Place the cell culture plate inoculated with the cell mixture in CO 2 Cultured in an incubator. The HAT medium containing 15% fetal bovine serum was used for the 1st to 4th day, the HT medium was used for the 5th to 10th day, and the RPM1-1640 medium containing 10% fetal bovine ser...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com