Bacillus coli periplasm cavity secretion type expression vector
An expression vector, Escherichia coli technology, applied in the use of vectors to introduce foreign genetic material, biochemical equipment and methods, applications, etc., can solve the problem of affecting correct conformation formation, increasing operating steps and production costs, and unfavorable foreign protein formation. Sulfur bridges, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0036] The present invention will be described in further detail below in conjunction with specific embodiments and with reference to the accompanying drawings. It should be understood that the embodiments in this specification are only for illustrating the present invention, but not limiting the scope of the present invention in any way.
Embodiment 1
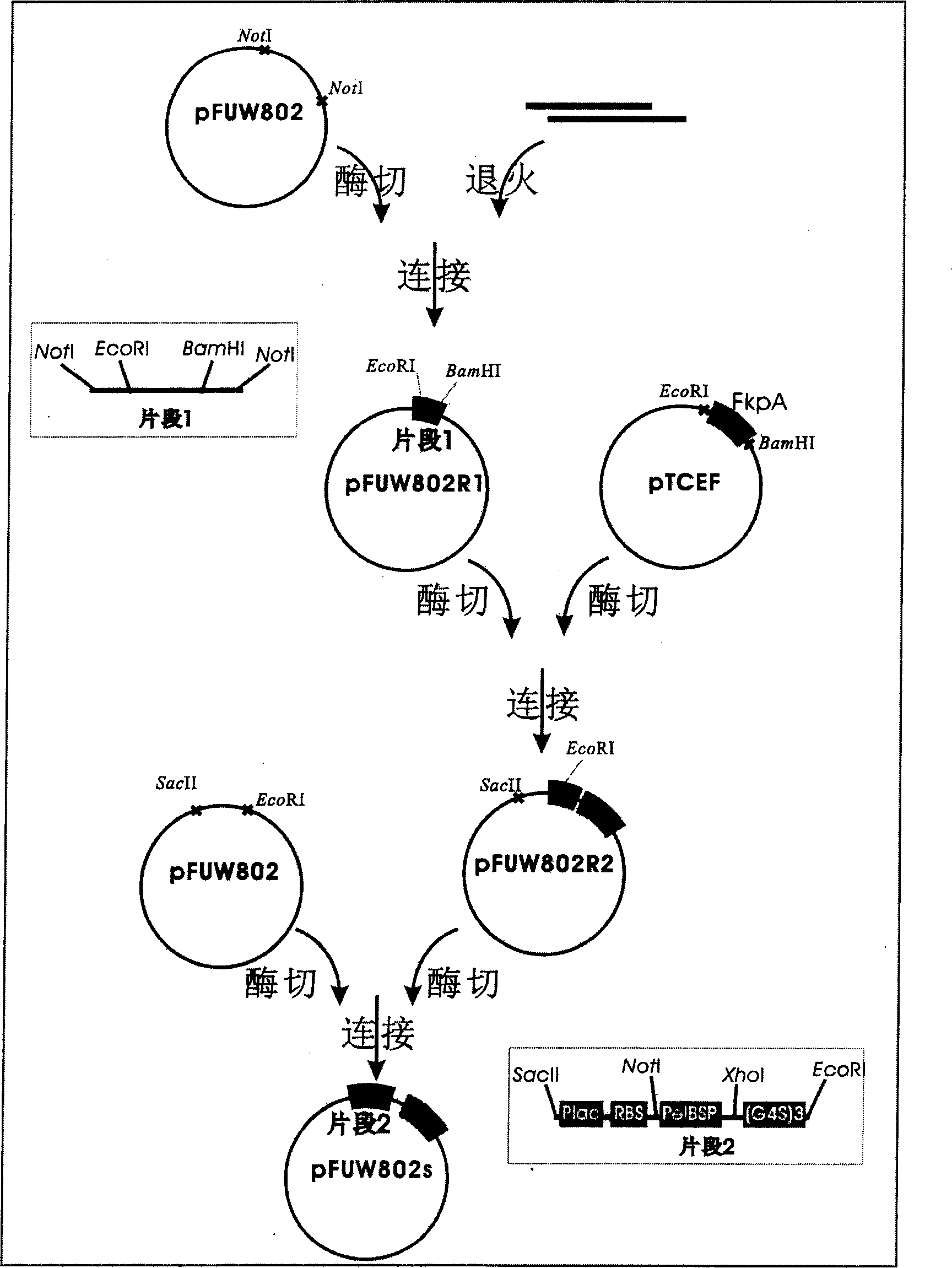
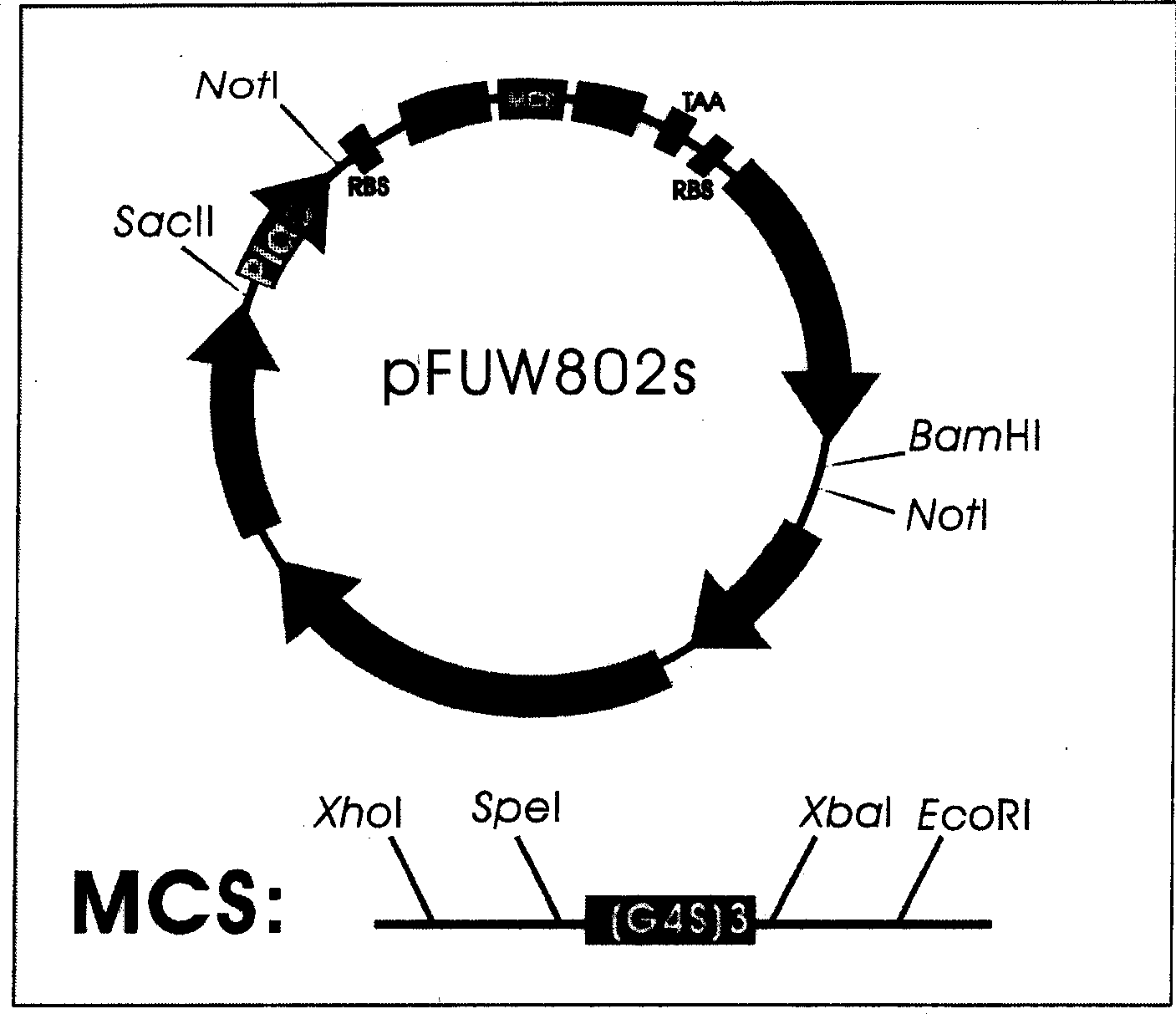
[0039] Example 1. Construction of the periplasmic cavity secreted expression vector pFUW802s (see the construction route figure 1)
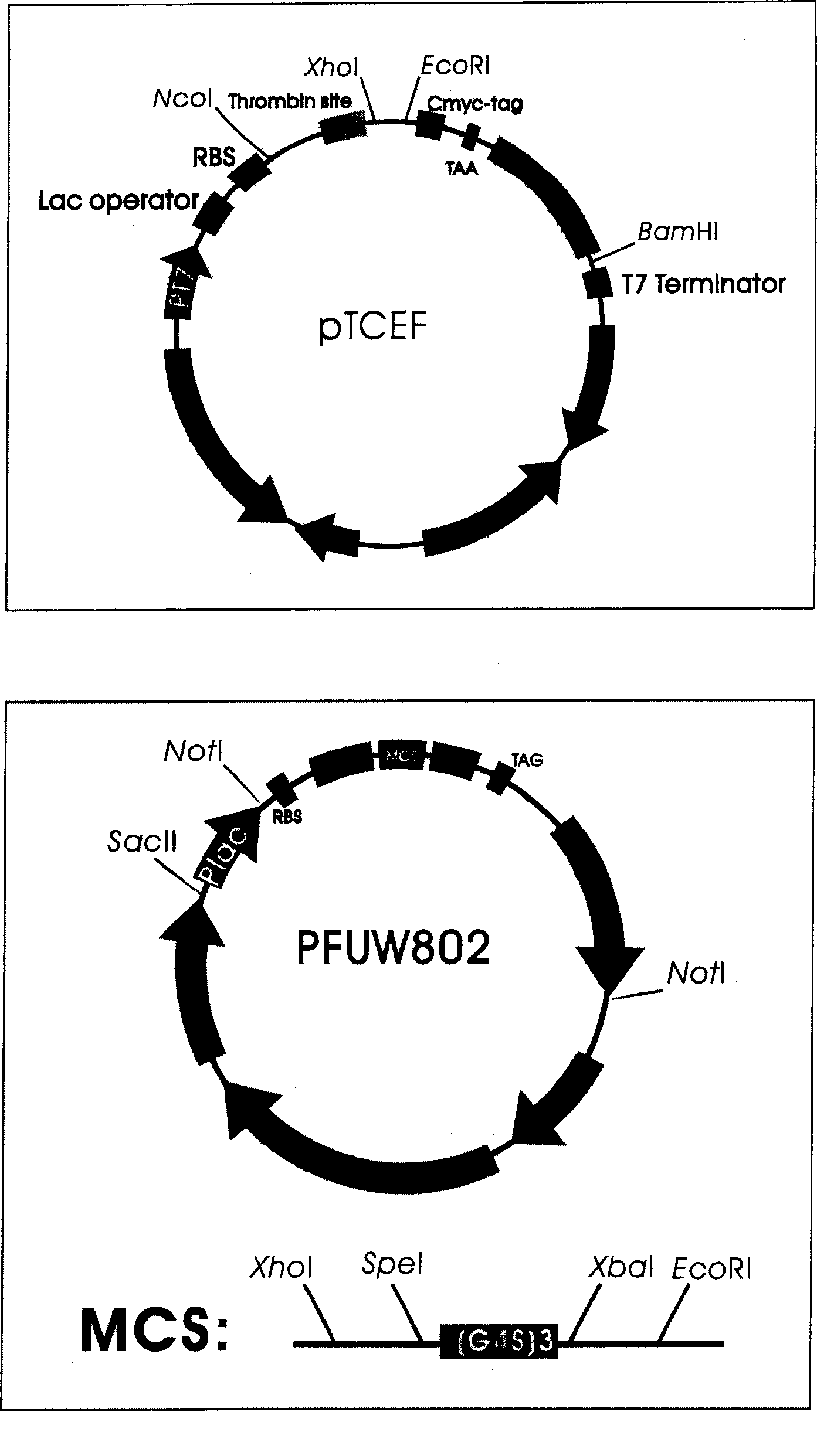
[0040] 1. Change the multiple cloning site of the bifunctional expression vector pFUW802.
[0041] according to Figure 1 Construction process, first design a pair of primers (primer 1: 5'-GGCCGCGAATTCAAATTCTATTTCGGATCCGGC-3' (SEQ ID NO: 2); primer 2: 5'-GGCCGCCGGATCCGAAATAGAATTTGAATTCGC-3' (SEQ ID NO: 3)), after annealing A double-stranded DNA fragment is formed, and the restriction site NotI is automatically formed at both ends of the fragment. The specific method is as follows: 1 μl each of primer 1 and primer 2, add 8 μl of water, and repeat the following reaction 10 times on a common PCR instrument: 94°C for 30 sec, 72°C for 30 sec. The reaction product was directly used as an exogenous fragment for the following ligation reaction.
[0042] About 1 μg of the vector pFUW802 was extracted using the plasmid mini-extraction kit from Shangha...
Embodiment 2
[0055] Example 2. Construction of pFUW802s / CEAscFv and expression identification (SDS-PAGE, Western Blot) and activity analysis (ELISA) of anti-CEA single-chain antibody
[0056] 1. Construction of pFUW802s / CEAscFv
[0057] The gene sequence of the anti-CEA single-chain antibody was excised from the intracellular soluble expression vector (pTCEF / CEAscFv) of the anti-CEA single-chain antibody constructed in our laboratory by XhoI / EcoRI double digestion reaction, and used as the foreign source of the following ligation reaction Fragment; pFUW802s and pFUW802 were also subjected to the same enzyme digestion reaction, and after recovering the large fragment, it was used as the carrier fragment for the following ligation reaction. The gene sequence of the anti-CEA single-chain antibody was obtained from reference (Koga H. et al., 1990). Digestion reaction mixture: about 1 μg carrier (10 μl), 2 μl 10× buffer, 1 μl XhoI and 1 μl EcoRI, 6 μl double distilled water. Reaction conditio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com