Polysaccharide, preparation and use thereof
A polysaccharide and glucose technology, applied in the field of polysaccharides, can solve problems such as limitations and insufficient attention to polysaccharides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
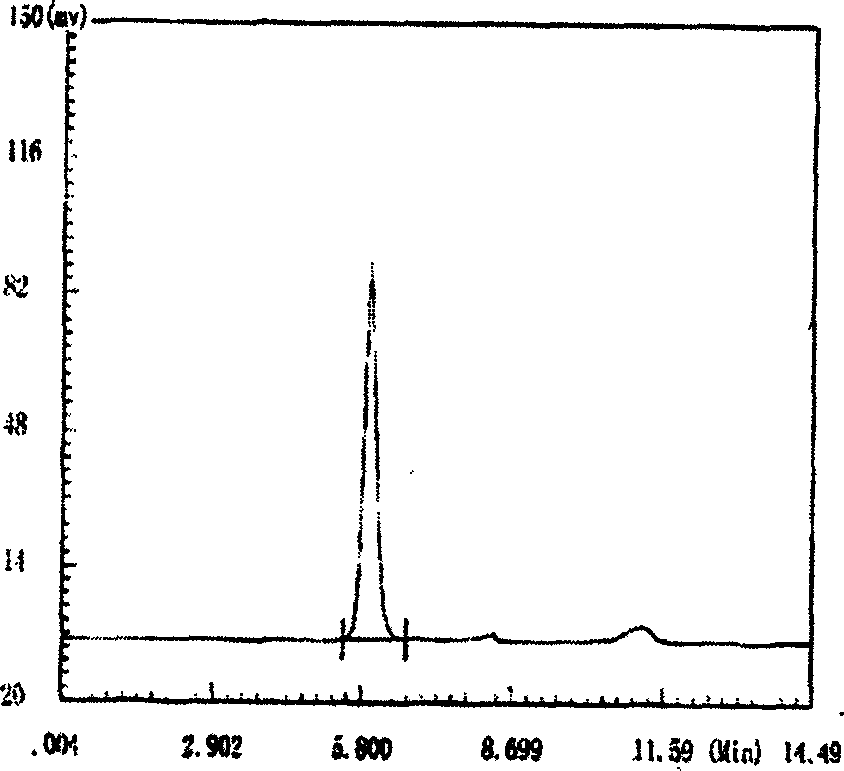
[0024] Example 1. Extraction, separation and purification, identification of polysaccharide YCP
[0025] Weigh 1000g of wet bacteria, add water, put it in a tissue masher and mash it, make up water to 2000mL, and extract in a water bath at 60°C for 20 hours. Stir intermittently, filter the residue with gauze, and centrifuge at 2000r.p.m. to remove the precipitate. Concentrate the extract to 500mL, add 1 / 4 volume of chloroform and 1 / 15 volume of n-butanol to the concentrated solution, vibrate vigorously, let stand to separate layers, remove the lower organic solvent layer and the middle layer, and keep the water layer. Repeat the extraction 2 times. The resulting solution was dialyzed against tap water for 24 hours. Concentrate the dialysate to 500 mL, gradually add an equal volume of ethanol, and place at 4°C for 2 hours. After centrifugation, the precipitate was washed successively with absolute ethanol, acetone, and ether, and the crude polysaccharide YCP was dried in vac...
Embodiment 2
[0029] Example 2. Extraction, separation and purification, identification of polysaccharide YCP
[0030] Weigh 1000g of wet fungus, add water, put it in a tissue masher and mash it, make up water to 3000mL, and extract in a water bath at 80°C for 12 hours. Stir intermittently, filter the residue with gauze, and centrifuge at 3000r.p.m. to remove the precipitate. Concentrate the extract to 1000mL, add 1 / 5 volume of chloroform and 1 / 25 volume of n-butanol to the concentrated solution, vibrate vigorously, let stand to separate layers, remove the lower organic solvent layer and the middle layer, and keep the water layer. Extraction was repeated 4 times. The resulting solution was dialyzed against tap water for 36 hours. The dialysate was concentrated to 1000 mL, and 3 times the volume of ethanol was gradually added, and left at 4°C for 6 hours. After centrifugation, the precipitate was washed successively with absolute ethanol, acetone, and ether, and the crude polysaccharide w...
Embodiment 3
[0032] Example 3. Extraction, separation and purification, identification of polysaccharide YCP
[0033] Weigh 1000g of wet bacteria, add water, put it in a tissue masher and mash it, make up water to 4000mL, and extract in a water bath at 90°C for 8 hours. Stir intermittently, filter the residue with gauze, and centrifuge at 4000r.p.m. to remove the precipitate. Concentrate the extract to 2000mL, add 1 / 7 volume of chloroform and 1 / 30 volume of n-butanol to the concentrated solution, vibrate vigorously, let stand to separate layers, remove the lower organic solvent layer and the middle layer, and keep the water layer. Extraction was repeated 5 times. The resulting solution was dialyzed against tap water for 48 hours. The dialysate was concentrated to 2000 mL, and 4 times the volume of ethanol was gradually added, and left at 4°C for 10 hours. After centrifugation, the precipitate was washed successively with absolute ethanol, acetone, and ether, and the crude polysaccharide w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com