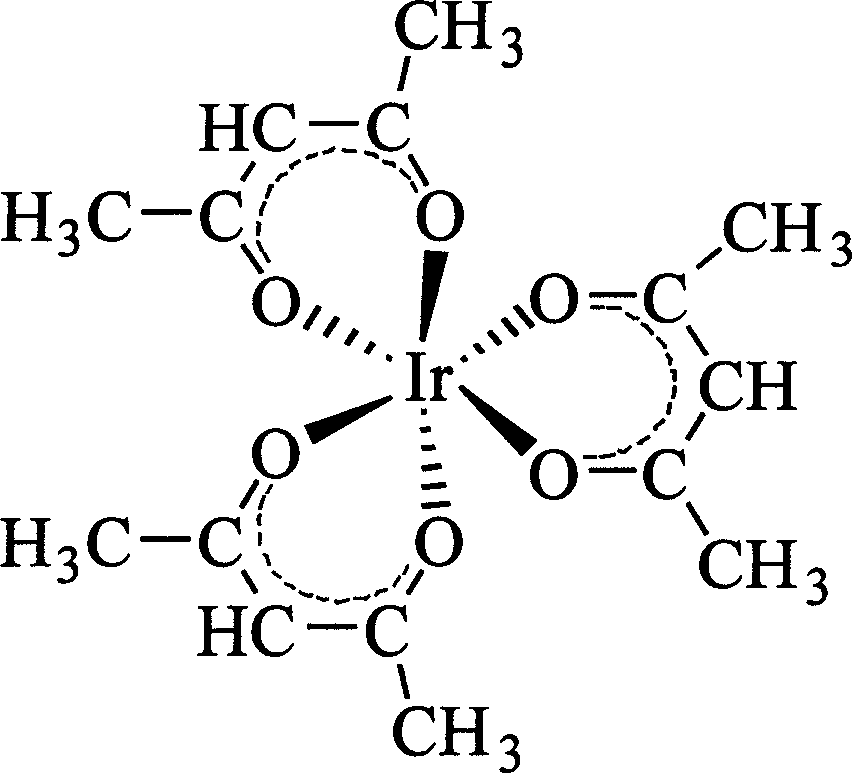
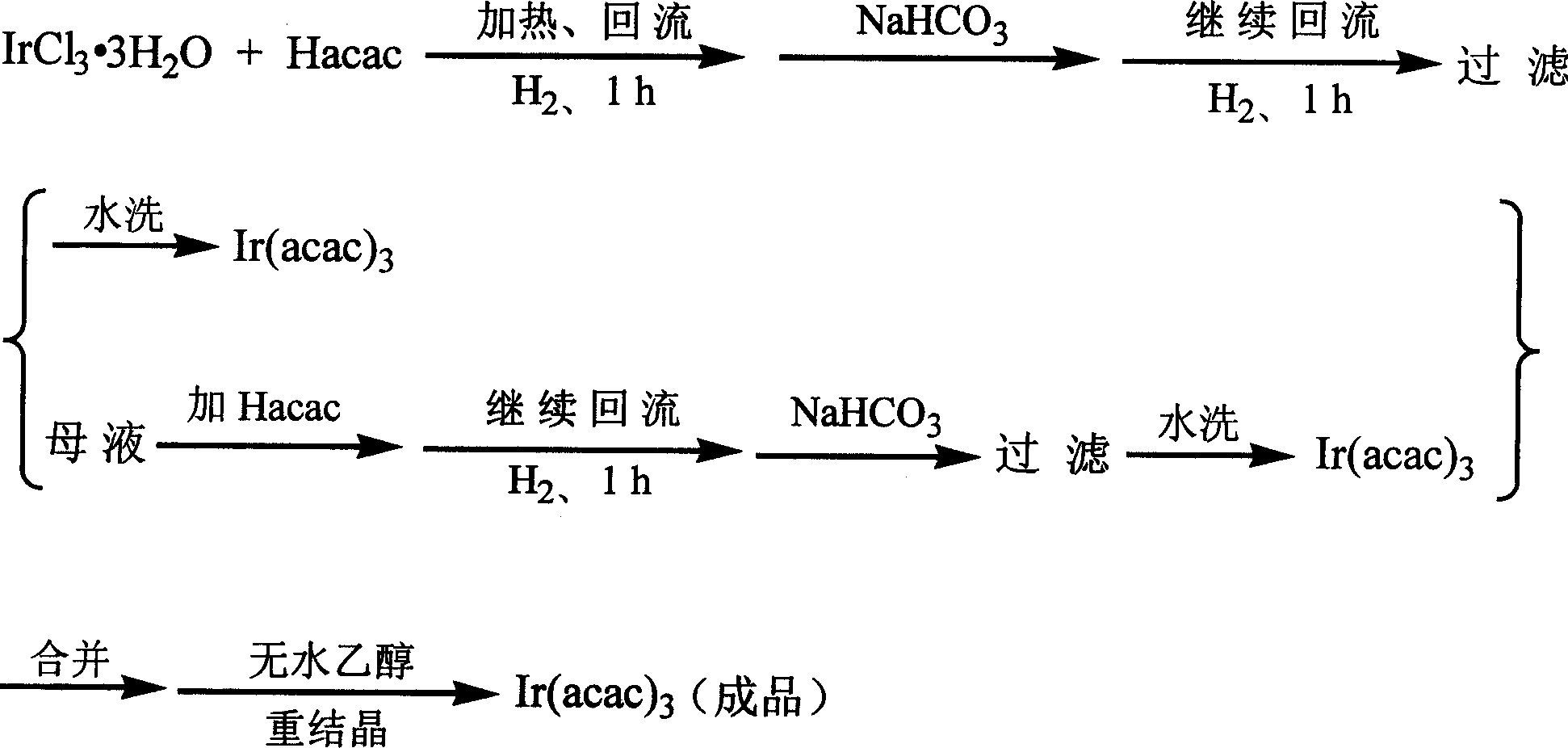
Method for synthesizing iridium (III) triacetylacetonate
A synthesis method and technology of acetylacetone, applied in the synthesis field of triacetylacetonate (iridium (iridium)), can solve problems such as complex process and low yield, and achieve the effects of promoting development, high social and economic benefits, and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Dissolve 10g of iridium(III) chloride trihydrate in 150mL of hot distilled water at 50°C, add 17.5mL of acetylacetone and 2.0g of ascorbic acid, stir and heat until boiling (boiling temperature is 96°C to 100°C), and reflux for 1 hour Slowly add saturated sodium bicarbonate solution (containing 6.8 g of sodium bicarbonate) dropwise, then reflux for 1.5 h, stop heating, cool, and filter with suction to obtain an orange-yellow product, namely iridium(III) triacetylacetonate, which is washed with water two to three times. Pour the filtrate back into the reactor, add 9.0 mL of acetylacetone, continue to stir and heat to reflux for 1 h, then slowly add saturated sodium bicarbonate solution (containing 3.5 g of sodium bicarbonate) dropwise, reflux for 1 h, cool, and suction filter to obtain the second batch of three Iridium(III) acetylacetonate, washed with water two to three times.
[0021] The iridium(III) triacetylacetonate obtained in the above steps was combined, purifie...
Embodiment 2
[0023] Dissolve 10g of iridium(III) chloride trihydrate in 150mL of hot distilled water at 50°C, add 17.5mL of acetylacetone, stir and start flowing hydrogen, then heat until boiling (boiling temperature is 92°C to 96°C), and reflux for 1.5h Then slowly add saturated sodium bicarbonate solution (containing 6.8 g of sodium bicarbonate) dropwise, then reflux for 1.5 h, stop heating until the reaction solution is cooled, then stop the hydrogen flow, and suction filter to obtain an orange-yellow product that is iridium triacetylacetonate ( III), wash with water two to three times. Pour the filtrate back into the reactor, ventilate with hydrogen, add 9.0mL of acetylacetone, continue to stir and heat to reflux for 1.5h, then slowly add saturated sodium bicarbonate solution (containing 3.5g of sodium bicarbonate) dropwise, reflux for 1h, cool down, stop the hydrogenation , Suction filtration to obtain the second batch of triacetylacetonate iridium (III), washed with water two to thre...
Embodiment 3
[0026]Dissolve 10g of iridium(III) chloride trihydrate in 150mL of hot distilled water at 45°C, add 17.5mL of acetylacetone and 2.0g of ascorbic acid, stir and start flowing hydrogen, then heat until boiling (boiling temperature is 92°C to 97°C) , after reflux for 2 hours, slowly add saturated sodium bicarbonate solution (containing 6.8 g of sodium bicarbonate), reflux for another 2 hours, stop heating until the reaction solution is cooled, then stop the hydrogen flow, and filter with suction to obtain an orange-yellow product that is triacetylacetonate Iridium(III), washed two to three times with water. Pour the filtrate back into the reactor, ventilate with hydrogen, add 9.0mL of acetylacetone, continue to stir and heat to reflux for 1.5h, then slowly add saturated sodium bicarbonate solution (containing 3.5g of sodium bicarbonate) dropwise, reflux for 1h, cool down, stop the hydrogenation , Suction filtration to obtain the second batch of triacetylacetonate iridium (III), w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com