Method for preparing electron transport / hole barrier material and its electro-glow parts
A device and red light technology, applied in the field of new electroluminescent materials, can solve the problems of organic light-emitting device performance attenuation, damage to film uniformity, and inability to fully guarantee the stability of form, so as to improve melting point, improve comprehensive performance, and electronic The effect of enhanced transmission capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
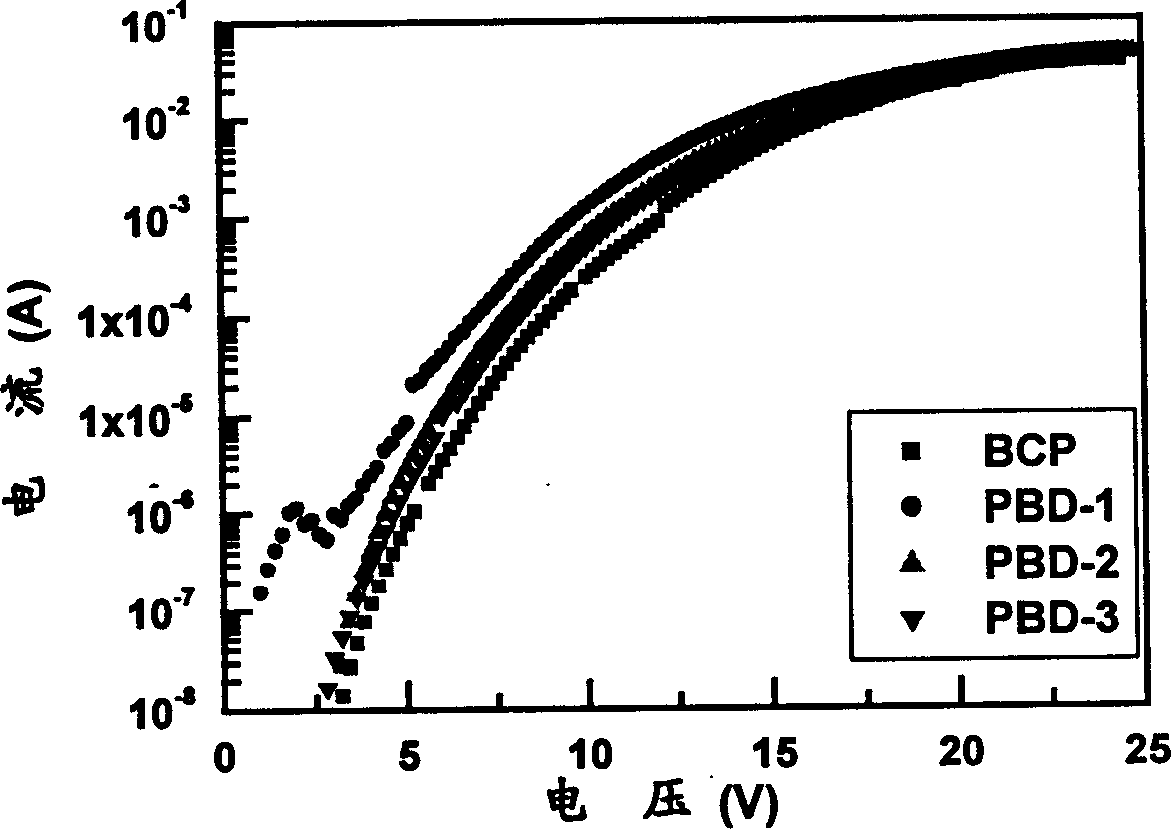
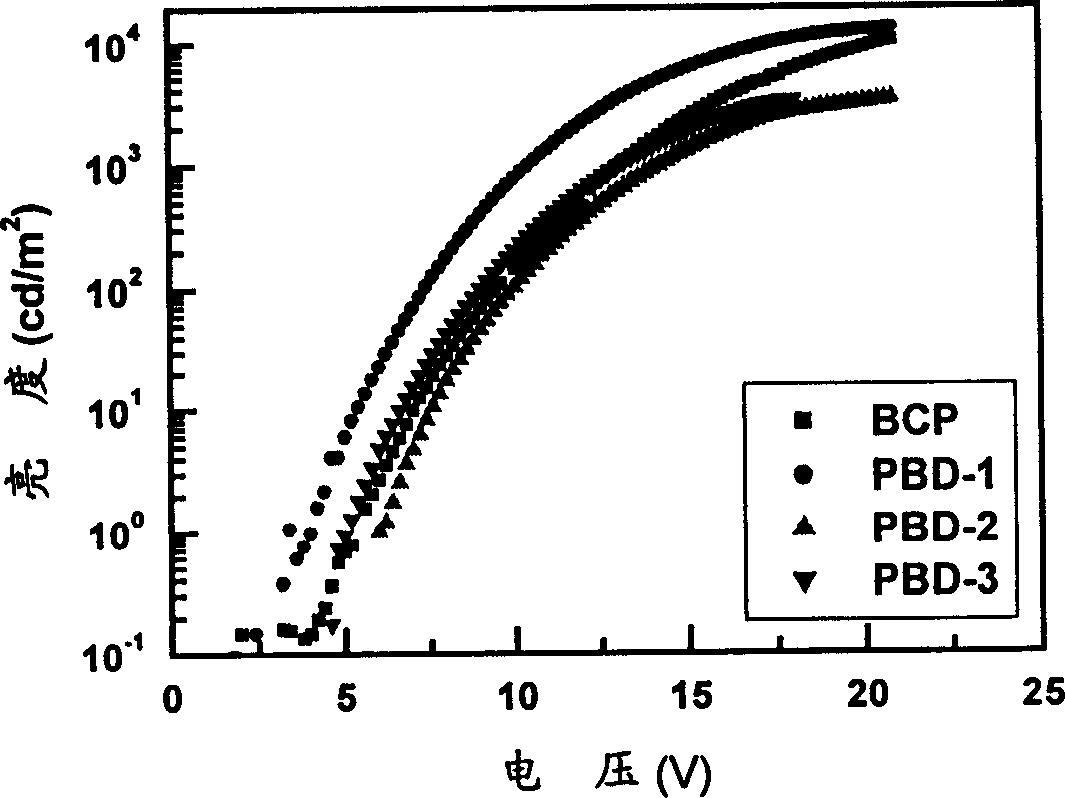
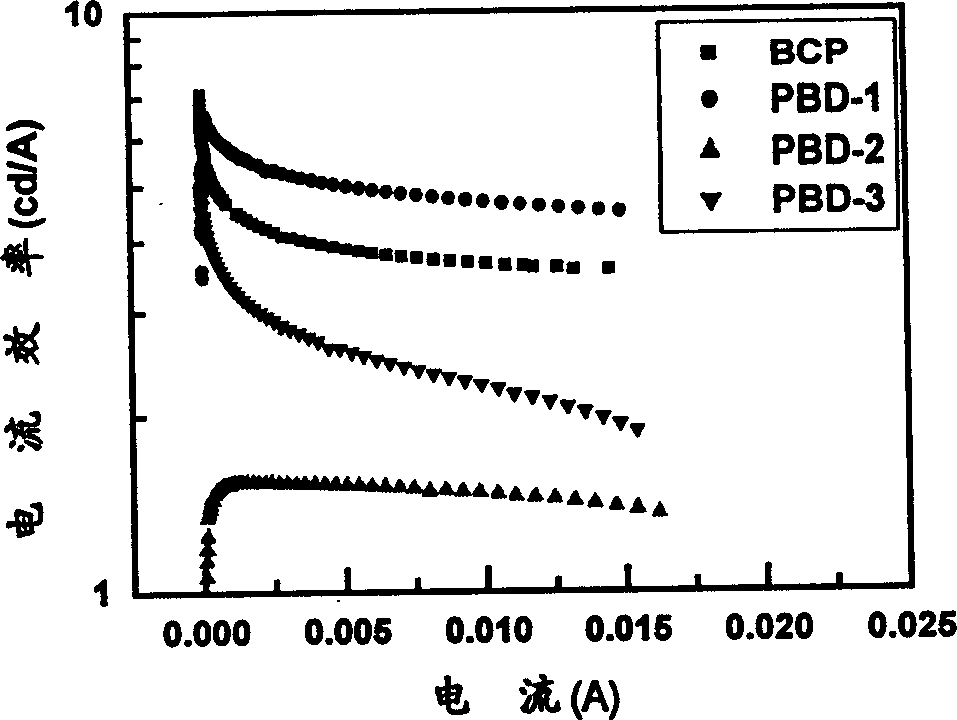
Image
Examples
Embodiment
[0042] In order to better understand the content of the patent of the present invention, the technical solution of the present invention will be further described below through specific examples and illustrations.
[0043] The example proceeds as follows:
example 1
[0044] Example 1, the preparation of 2,5-bis-4'-(4"-trifluoromethylbiphenyl)-1,3,4-oxadiazole
[0045] Ethyl 4-bromobenzoate (20 mL) and hydrazine hydrate (27.5 mL) were refluxed in methanol (124 mL) for 16 hours, cooled to room temperature and crystallized to obtain 25.3 g of needle-like white crystals of 4-bromobenzohydrazide, (87% yield). 1 H NMR (500 MHz, DMSO, ppm): δ 7.7 (d, 2H), δ 7.6 (d, 2H), δ 9.8 (s, 1H), δ 4.54 (s, 2H).
[0046]4-Bromobenzoyl chloride (11g) and 4-bromobenzoyl hydrazide (10.5g) were refluxed in pyridine (200mL) which had been distilled in advance for about 10h (nitrogen gas flowed during the reaction), and the solution was cooled to room temperature and crystallized Flaky white crystals were obtained, and 14.5 g of the product 4-bromobenzoic acid-N-(4-bromobenzoyl)hydrazine was obtained after suction filtration (97% yield). 1 H NMR (500MHz, CDCl 3 , ppm): δ10.64 (s, 2H), δ7.85 (d, 4H), δ7.76 (d, 4H).
[0047] 4-Bromobenzoic acid-N-(4-bromobenzoyl...
example 2
[0050] Example 2, the preparation of 2,5-bis-4'-(4"-fluorobiphenyl)-1,3,4-oxadiazole
[0051] 2,5-bis-(4-bromophenyl)-1,3,4-oxadiazole (0.5 g) and 4-fluorophenylboronic acid (0.45 g) were stirred and heated in 35 mL of toluene, and N 2 Protection, adding catalyst Pd(PPh 3 ) 4 (catalyst amount) and K 2 CO 3 Solution 3mL (2mol / L), refluxed overnight.
[0052] The reaction mixture was washed with an appropriate amount of water, and the oil layer was washed with anhydrous MgSO 4 After drying, rotary evaporation and recrystallization gave 0.46 g of white solid product with a yield of 85%. F.W.410, m.p.259.3°C, 1 H NMR (400MHz, CDCl 3 , ppm): δ7.18 (d, 4H), δ7.62 (m, 4H), δ7.72 (d, 4H), δ8.22 (d, 4H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com