Bacterin of tabling gene of E type hepatitis virus
A technology of hepatitis E virus and chimeric gene, which is applied in gene therapy, antiviral agent, genetic engineering, etc., and can solve problems such as the development of HEV chimeric gene vaccine that has not yet been developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
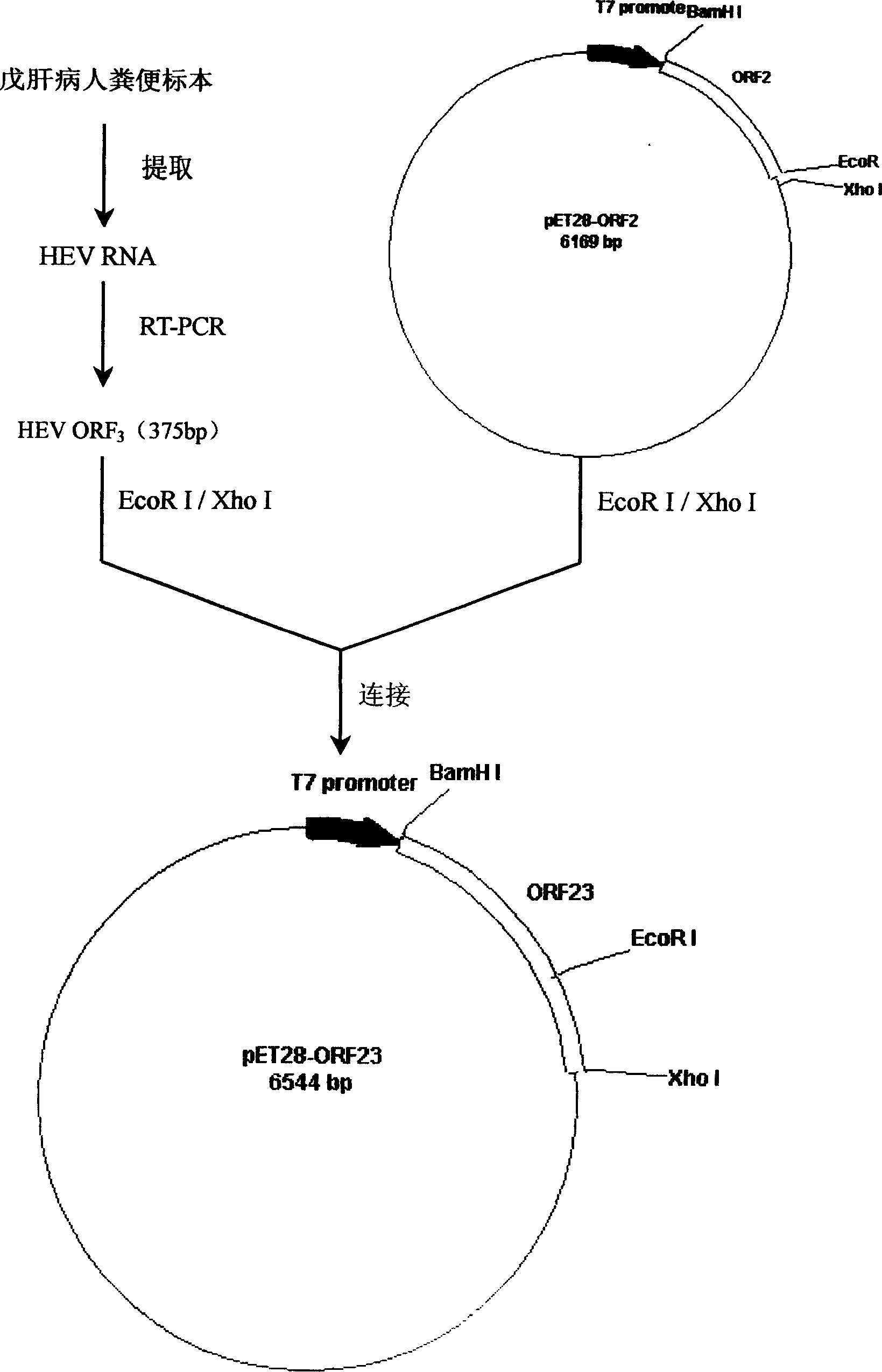
[0072] Example 1 :Construction of prokaryotic expression plasmid containing HEV ORF23 chimeric gene
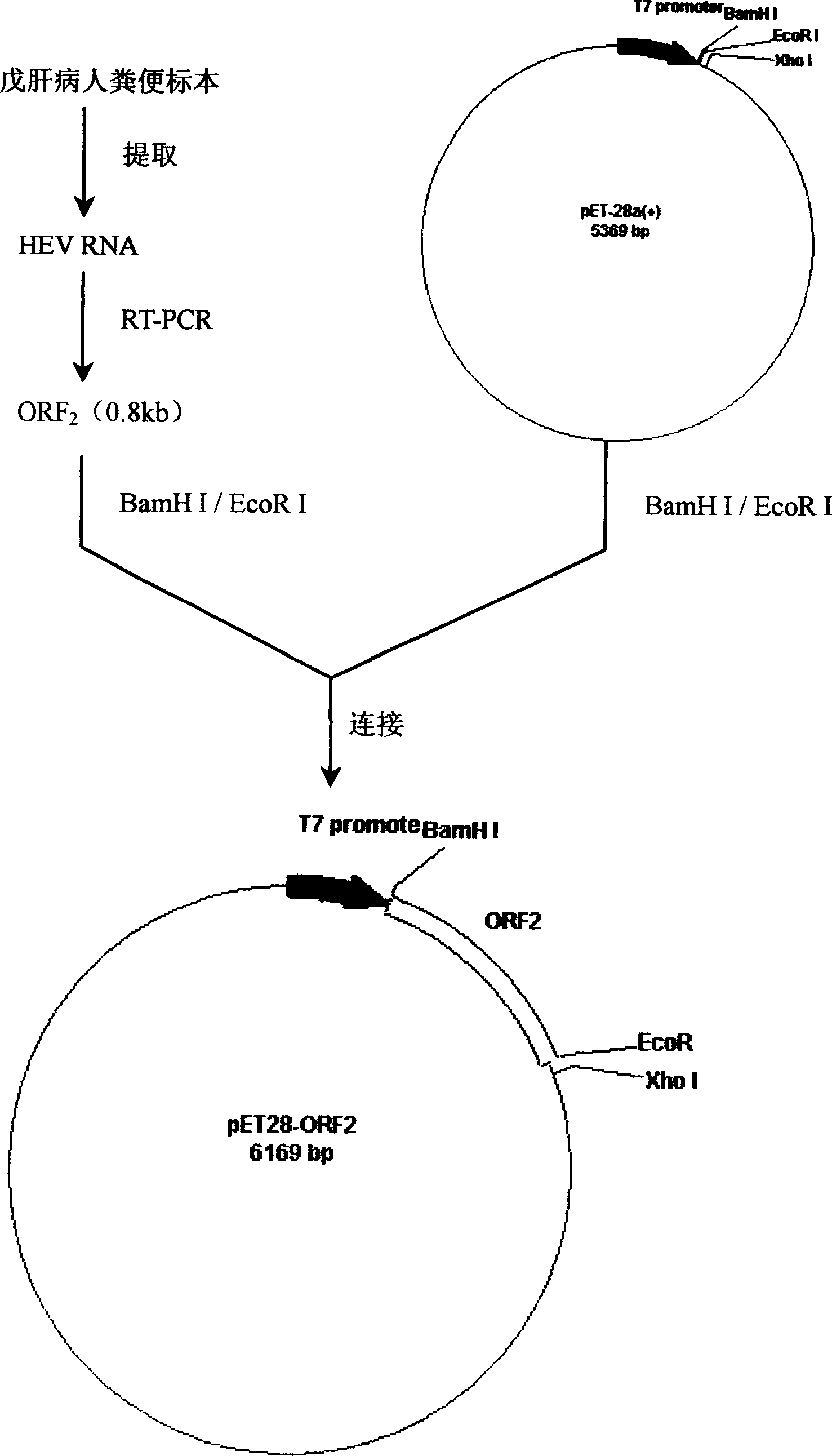
[0073] Using the stool of hepatitis E patients as specimens, the viral RNA was extracted according to conventional methods, and the HEV ORF2 gene was amplified by RT-PCR using primers 1 and 2, and the HEV ORF3 gene was amplified by RT-PCR using primers 2 and 4. Reverse transcription reaction (RT) conditions were: 42°C for 1 hour; PCR conditions were: 94°C for 1 minute, 52°C for 1 minute, 72°C for 2 minutes, a total of 35 cycles. After the above PCR product was purified, it was digested with BamHI / EcoRI and the ORF2 gene fragment was inserted into pET28a + In the plasmid, obtain pET28-ORF2 (see attached figure 2 ), then cut with EcoRI / XhoI and insert the ORF3 gene fragment into pET28-ORF2 plasmid to obtain the chimeric recombinant plasmid pET28-ORF23 (see attached image 3 ).
[0074] Primer 1: 5’GTC GGA TCC ATG CAG CTG TTC TAC TCC CGT 3’
[0075] Primer 2: 5’GTC GAA TTC AAC TCC...
Embodiment 2
[0079] Example 2 :Construction of yeast expression recombinant plasmid containing HEV ORF23 chimeric gene
[0080] Using the chimeric recombinant plasmid pET28-ORF23 as a template, PCR amplification was performed with primers T1 and T2. The PCR conditions were: 94°C for 1 minute, 52°C for 1 minute, 72°C for 2 minutes, 33 cycles. After the above PCR product was purified, it was digested with Kpn I / Xba I and inserted the ORF23 gene fragment into the yeast expression recombinant plasmid pPICZαA to obtain the chimeric recombinant plasmid pPICZαA-ORF23 (see attached Figure 4 ).
[0081] Primer T1: 5’GAC TGG GTA CCC AGC TGT TCT ACT CTC GT 3’
[0082] Primer T2: 5’GTA CTC TAG ACA GCG GCG CGG TCC CAG C 3’
[0083] Expression and purification of chimeric protein ORF23: The chimeric recombinant plasmid pPICZαA-ORF23 was linearized with Sac I to linearize the plasmid vector, and the linearized plasmid DNA was transferred into yeast cells by electrotransfer. After electroporation, the cell...
Embodiment 3
[0084] Example 3 :The construction of chimeric gene vaccine and the experiment of immunizing mice
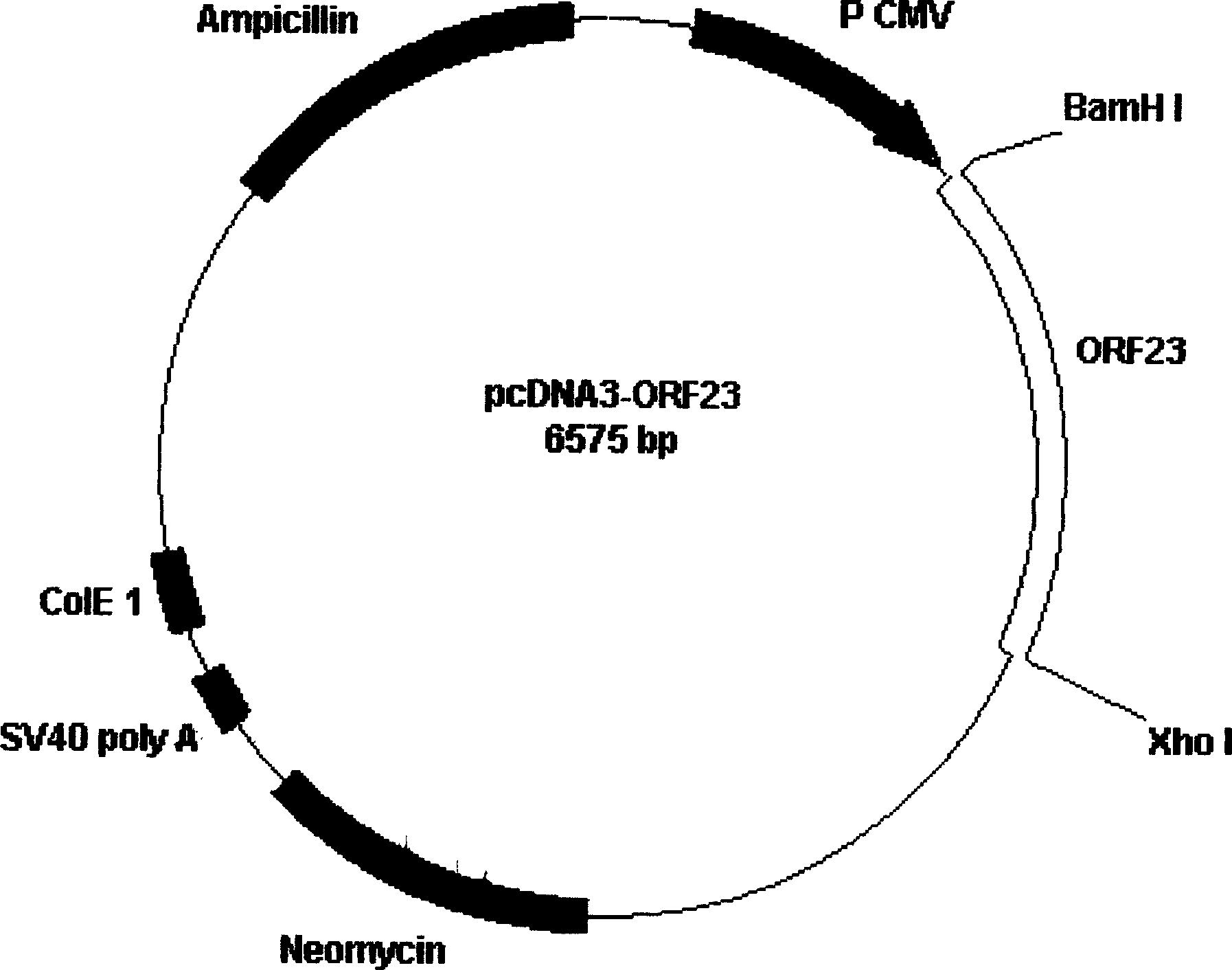
[0085] With BamHI / XhoI double enzyme digestion, the HEV ORF23 gene fragment was cut from the recombinant plasmid pet28-ORF23, and then inserted into pcDNA 3 In the plasmid, obtain the recombinant plasmid pcDNA 3 -ORF23 (see attached figure 1 , Attached Figure 5 ). The recombinant plasmid was used as a chimeric gene vaccine to immunize Blab / C mice. The immunization route was intramuscular injection. The injection doses were 100ug and 200ug, once every 3 weeks, for a total of 2 injections. Two weeks after each injection, the mouse serum was taken to test for antibodies, and the T cell proliferation response of the mice was tested after the last injection. The results showed that after two immunizations, the mice all produced HEV-specific antibodies and produced a significant T cell proliferation response. It shows that the recombinant plasmid can be used as a HEV chimeric gene vacc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com