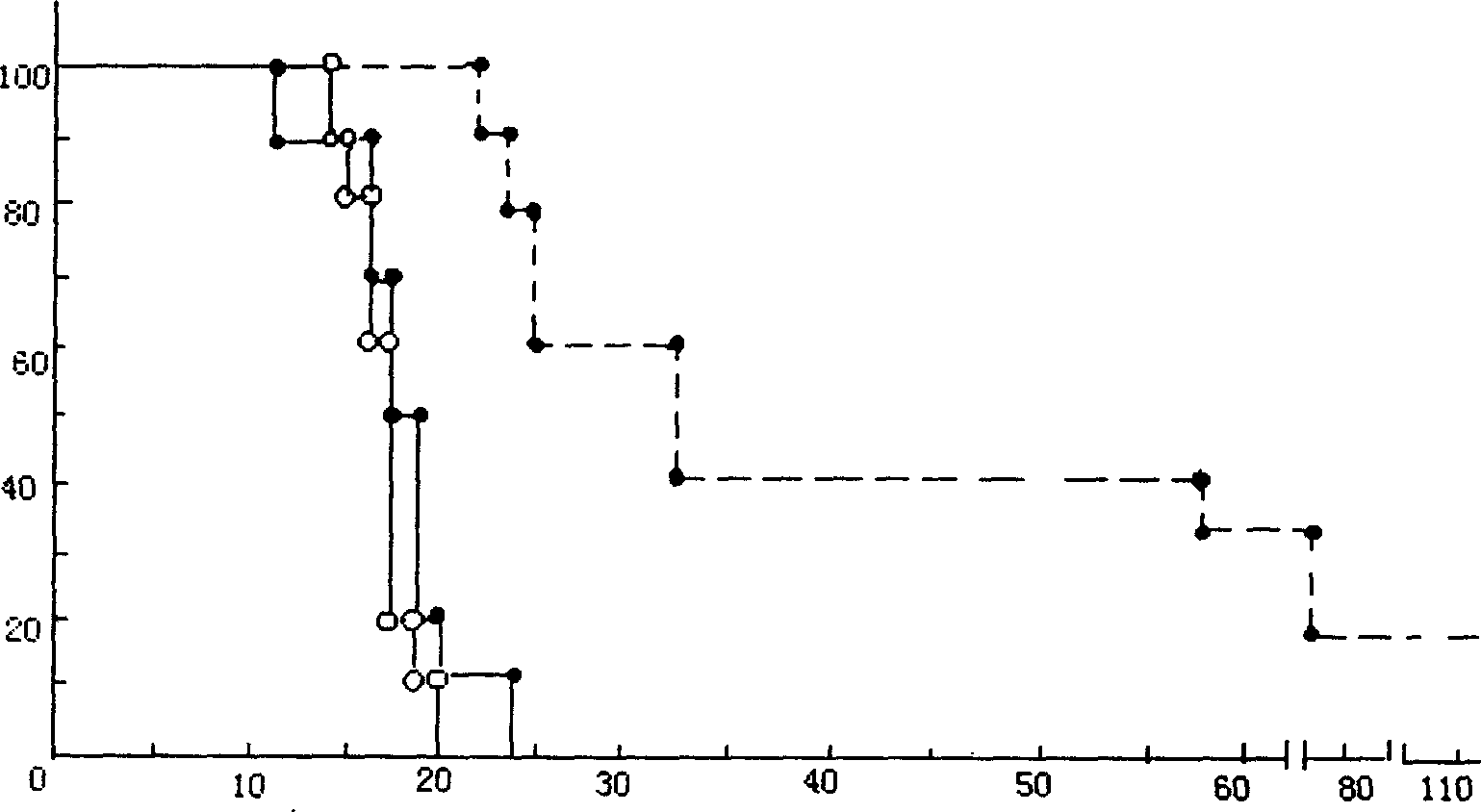
Carmustine slow-release tablet and its preparation method
A slow-release film and a slow-release film technology are applied in the field of carbazide slow-release film and its preparation, which can solve the problems of inability to specifically act on tumor sites, large toxic reactions, etc., to avoid peaks and troughs, reduce painful effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0033] Polylactic acid-chloroform (CHCL 3 ) solution: first mix 0.05kg of 20,000 molecular weight L-polylactic acid and 70,000 molecular weight L-polylactic acid into solid polylactic acid powder; put 0.1kg of solid polylactic acid powder into a round bottom flask, add chloroform Solution 0.9kg, put the flask in a matching electric heating mantle, install a condensing device at the mouth of the bottle, turn on the power (connected to an adjustable transformer), heat slowly, adjust the voltage when boiling to keep it slightly boiling for 1 hour, and then disconnect the power Turn off heat.
[0034] When the temperature of the solution drops to room temperature, add 0.01 kg of carmustine and shake gently to completely dissolve the carmustine.
[0035] Casting film: In the ultra-clean bench, pour the polylactic acid-carmustine-chloroform solution prepared above into several polyethylene molds to make a film containing 10% carmustine with a diameter of about 13-15mm , a diaphrag...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com