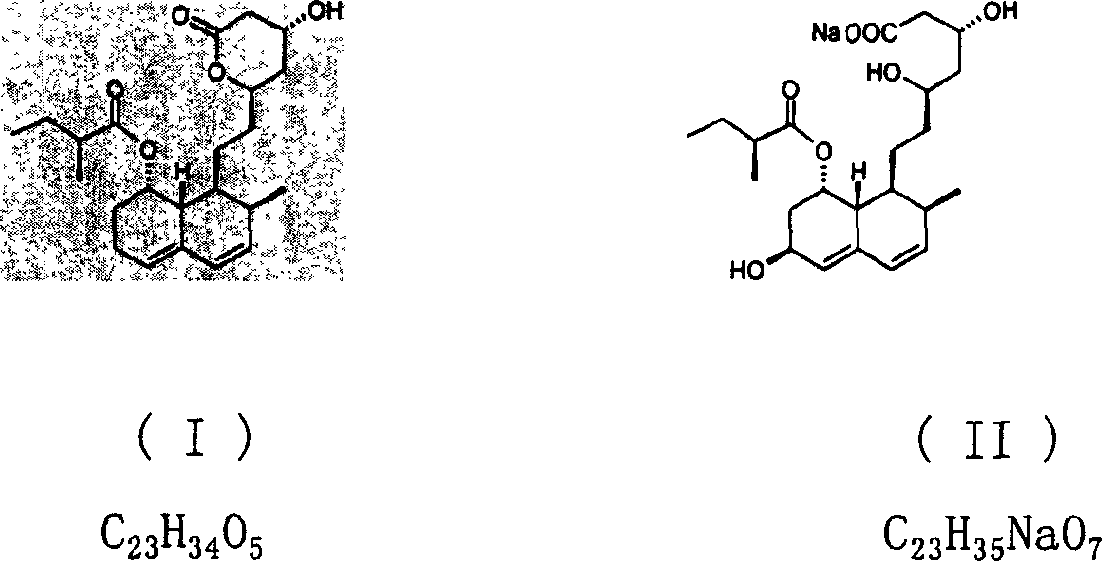
Purification method of pravastiatin sodium
A technology of pravastatin sodium and purification method, which is applied in chemical instruments and methods, organic chemistry, preparation of organic compounds, etc., and can solve problems such as not being able to be removed well, endangering the health of production operators, and low concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Take 2.5 liters of the microbial transformation solution containing pravastatin sodium and detect by HPLC, the content of pravastatin sodium is 1.5 g / L (total 3.75 g), the HPLC purity is 65%, and the pH of the transformation solution is adjusted by sodium hydroxide 7.0, then add 20 grams of diatomaceous earth and stir for 15 minutes, then filter on a Buchner funnel lined with filter paper and collect the filtrate.
[0020] The filtrate obtained in the previous step was loaded onto a chromatography column containing 500 ml of XAD-16HP resin, and the sample flow rate was 0.5 bed volume per hour. After loading the sample, wash 2 volumes of resin with water at a flow rate of 1 column bed volume per hour. After washing, use 700 ml of methanol as the eluent, and the eluent flow rate is 1 column bed volume per hour. Collect the fraction containing pravastatin sodium and mix. The collected liquid was tested by HPLC, and the content of pravastatin sodium was 3.5 g (yield 93.3%) and ...
Embodiment 2
[0022] Take 15 liters of the microbial transformation solution containing pravastatin sodium and detect it by HPLC. The content of pravastatin sodium is 1.25 g / L (18.75 g in total), and the purity determined by HPLC is 72%. The transformation solution is adjusted by sodium hydroxide The pH is 7.2, then 80 grams of perlite is added and stirred for 15 minutes, and then filtered on a Buchner funnel with filter paper to collect the filtrate.
[0023] The filtrate obtained in the previous step was loaded onto a chromatography column containing 2.5 liters of HP-20 resin at a flow rate of 0.5 bed volume per hour. After loading the sample, wash 2 volumes of resin with water at a flow rate of 1 column bed volume per hour. After washing, use 4 liters of ethanol as the eluent, and the flow rate of the eluent is 1 column bed volume per hour. Collect the fractions containing pravastatin sodium and mix them. The collected solution was tested by HPLC, and the content of pravastatin sodium was 17...
Embodiment 3
[0025] Take 5 liters of the microbial transformation solution containing pravastatin sodium and detect it by HPLC. The content of pravastatin sodium is 1.25 g / L (total 6.25 g), and the purity determined by HPLC is 72%. The transformation solution is adjusted by sodium hydroxide The pH is 7.2, then 80 grams of perlite is added and stirred for 15 minutes, and then filtered on a Buchner funnel with filter paper to collect the filtrate.
[0026] Put about 5 liters of the filtrate obtained in the previous step in a 10 liter stainless steel bucket, add 1000ml XAD-16 resin, stir at room temperature for 120 minutes, then pour the filtrate containing the resin on a Buchner funnel covered with filter paper for filtration, and discard the obtained filtrate To remove, the resin is loaded into the chromatography column, washed with 1000 ml of water, and eluted with 1600 ml of ethanol after washing, to collect the fraction containing pravastatin sodium. The collected solution was tested by HPLC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com