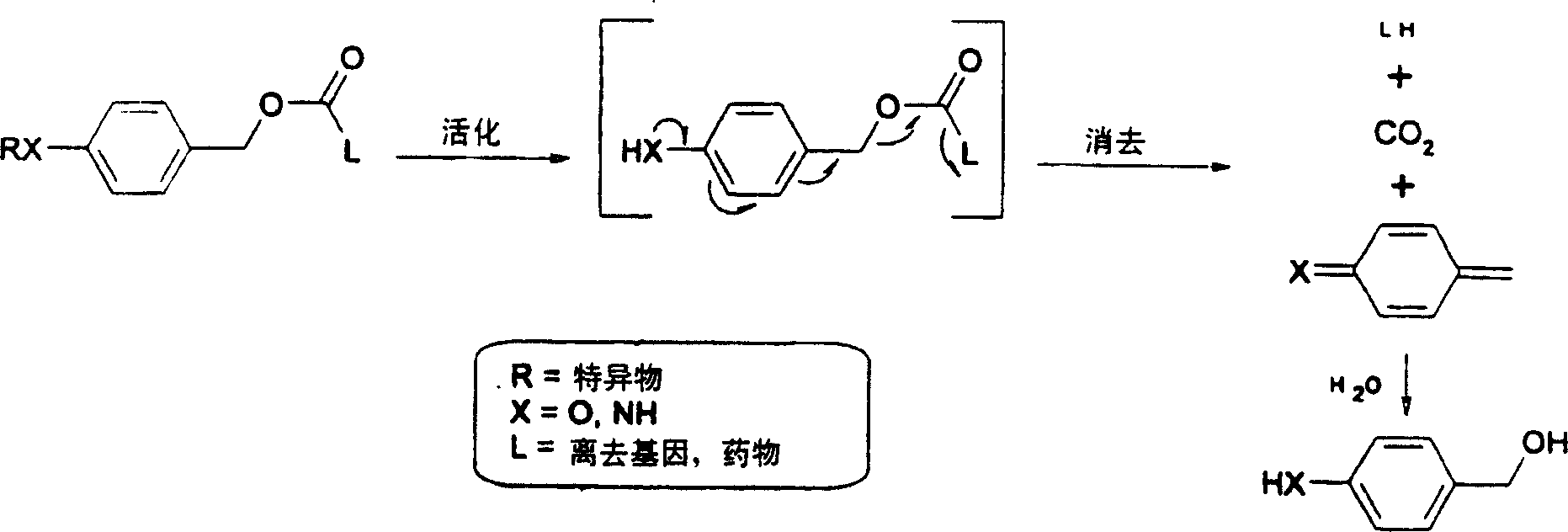
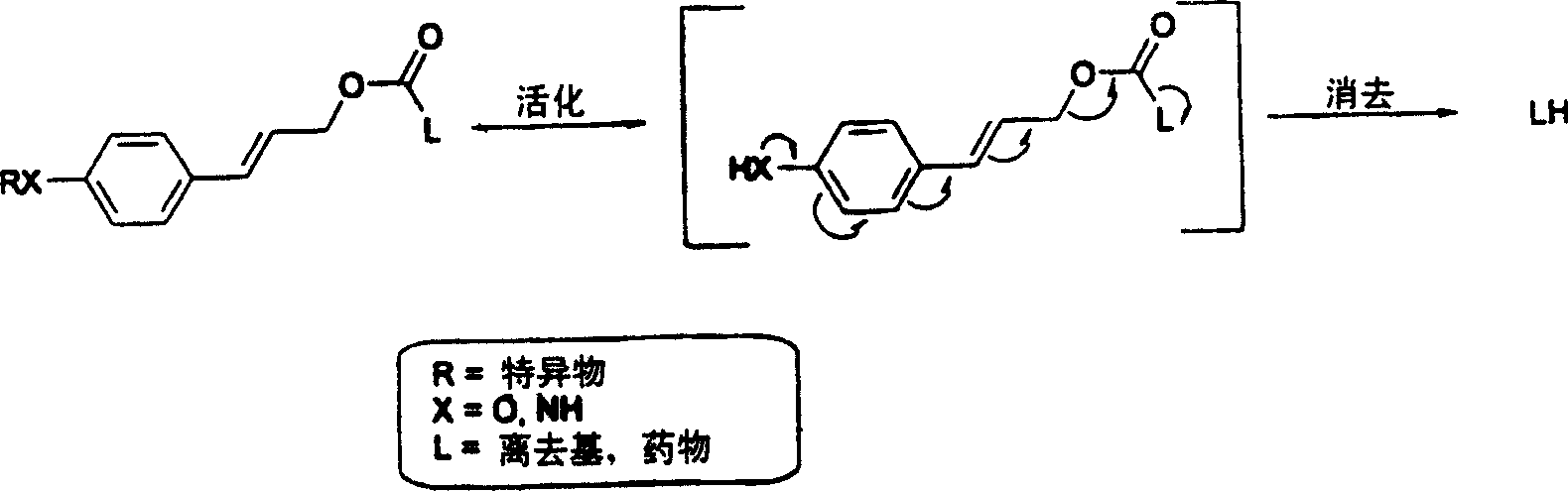
Elogated and multiple spacers in activatible prodrugs
A compound and alkyl technology, applied in the field of drug prodrugs, can solve problems such as reducing tumor cell drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Synthesis of 2′-[4-nitrocinnamyl carbonate]-paclitaxel 1.
[0115] To a solution of 200 mg (1.12 mmol, 4.8 eq) of 4-nitrocinnamyl alcohol in dry dichloromethane / tetrahydrofuran under an argon atmosphere were added pyridine (94 μl, 5.0 eq) and 4-nitrophenyl Chloroformate (236 mg, 5.0 equiv). The reaction mixture was stirred at room temperature for 12 hours. The mixture was cooled to 0° C. and a catalytic amount of DMAP, a few drops of triethylamine and 200 mg of paclitaxel (1.0 eq.) were added. The reaction mixture was stirred at room temperature for 12 hours. The solvent was evaporated and the residual solid was dissolved in dichloromethane. The organic layer was washed thoroughly with saturated sodium bicarbonate solution, 0.5N potassium bisulfate and brine, and dried over anhydrous sodium sulfate. After solvent evaporation, the residual yellow oil was purified by column chromatography (ethyl acetate-hexane; 1:1) to afford 144 mg of 1 (58%). M.P.151℃;
[0116] ...
Embodiment 2
[0118] Principles of 1,8-elimination: chemical reduction of nitrocinnamyl carbonate 1
[0119] 36 mg of 2'-[4-nitrocinnamyl carbonate]-paclitaxel 1 was dissolved in 8 ml methanol and 2 ml acetic acid. A catalytic amount of zinc dust was added and the resulting red mixture was stirred for 12 hours. Dichloromethane was added and the organic layer was washed with saturated sodium bicarbonate, 0.5N potassium bisulfate, brine and water, and dried over anhydrous sodium sulfate. After evaporation of the solvent, the residual yellow film was purified by column chromatography (ethyl acetate-hexane; 2:1) to obtain 28 mg of paclitaxel (via 300 MHz 1 H-NMR confirmed) and 4 mg of unreacted starting compound. When the compound was stirred under the same conditions in the absence of zinc powder, no paclitaxel was formed, suggesting that reduction of the nitro group by zinc resulted in the release of paclitaxel.
Embodiment 3
[0121] Synthesis of Aloc-D-Ala-Phe-Lys(Aloc)-OH 9
[0122] Step a: Synthesis of Fmoc-Phe-Lys(Boc)-OBu 4
[0123] To a solution of 2.50 g of Fmoc-Phe-ONSu 2 (ONSu=N-hydroxysuccinimide) (5.16 mmol) in dry dichloromethane at 0 °C in an argon atmosphere was added 0.791 ml of triethylamine (1.1 eq. .) and 1.92 g H-Lys(Boc)-OBu.HCl3 (1.1 eq.). The reaction mixture was stirred at room temperature for 5 hours, then dichloromethane was added and the organic layer was washed with the following solution: 10% citric acid, saturated sodium bicarbonate and water. The organic layer was dried over anhydrous sodium sulfate and evaporated. The resulting white solid 4 (3.08 g, 89%) was used directly without further purification. M.P.93℃; 1 H-NMR (300MHz, CDCl 3 ): δ1.10-1.90 (m, 24H, 6CH 2 -Lys and 18 tert-butyl), 3.06 (m, 2H, N-CH2 -Lys and benzyl), 4.19 (t, 1H, Fmoc), 4.25-4.55 (m, 4H, 2 Fmoc and 2 Hα), 7.19-7.78 (m, 13H, aromatic) ppm; MS (FAB) m / e 672(M+H) + , 694(M+Na) + ;C 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com