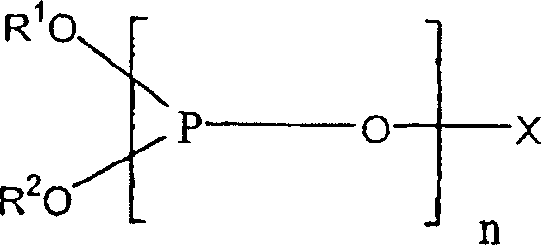
Production of alkyl 6-aminocaproate
A technology for alkyl aminocaproate and alkyl cyanovalerate, applied in the field of producing alkyl 6-aminocaproate and/or caprolactam
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Oxidative esterification of formylvaleronitrile to methyl cyanovalerate
[0074] This example demonstrates that formylvaleronitrile can be converted to methyl cyanovalerate (MCV) in one step with an increase in selectivity towards the linear isomer.
[0075] Contain the sample of the solution of 2.3g formyl valeronitrile (84.4% 5FVN, 3.7% 4FVN and 11.8% 3FVN), 10g methyl alcohol and 0.5g o-dichlorobenzene (GC internal standard) in 10wt% solid catalyst (as shown in table 1 ) in the presence of 50°C and 500 psig air pressure for 1 hour. The product was analyzed by gas chromatography with a Restex(R)-5 amine column (15 m x 0.25 mm). The conversion ratio is shown in Table 1. FVN linearity in the table = 100*5FVN / (3FVN+4FVN+5FVN). MCV linearity = 100*M5CV / (M3CV+M4CV+M5CV).
[0076] Table 1
[0077] PD 4 TeZnPbPd 4 TeZnPbBiPd 4 TeZ
[0078] 3FVN (mole%) 5.7 7.8 5.8
[0079] 4FVN(mole%) 1.7 2.1 1.8
[0080] 5FVN (mole%) 34.7...
Embodiment 2
[0088] Separation of linear and branched methyl cyanovalerate
[0089] This example demonstrates that branched isomers can be removed in a commercially viable distillation process (overhead pressure 10 Torr).
[0090] 300 g of mixed methyl cyanovalerate was used as the feed to a batch distillation column with 20 1 inch trays in a 500 ml volume Oldershaw still. GC analysis found that the feed composition was 14.3% M4CV, 7.7% M3CV and 75.9% M5CV, and the rest was mainly methanol acetal and 5FVN. At 10 torr (1.3×10 -3 MPa) pressure, start distillation under total reflux, and the condenser temperature is 0°C. Low boilers leave the distillation column mainly through the total reflux condenser. Under total reflux, the distillation column reached a steady state with a kettle temperature of 150°C and a tower top temperature of 102°C. The distillate was then collected into several 5 ml fractions with a vapor splitting head at a reflux ratio of 100:1. During the collection of 13 fr...
Embodiment 3
[0092] with Raney Synthesis of methyl 6-aminocaproate with cobalt catalyst
[0093] This example demonstrates methyl 5-cyanovalerate in Raney In the presence of cobalt, it can be hydrogenated to 6-aminocaproic acid methyl ester.
[0094] Charge 26.0 g methanol and 0.5 g Raney in a 100 ml stainless steel high pressure stirred reactor (Parr reactor) Cobalt 2724 (W.R. Grace Company). A cup was then secured to the top of the reactor, pressure tested with 100 psig nitrogen, and purged with hydrogen. It was then pressurized to 250 psig with hydrogen and heated to reaction temperature (75° C.) with constant stirring. 10.23 g of methyl 5-cyanovalerate (M5CV), 0.5 g of 1-methyl-2-pyrrolidone (internal standard) and 5.0 g methanol. The pressure in the reactor was then raised to the desired level (500 psig 3.5 MPa) and maintained at this level throughout the experiment (1.2 hours). During the reaction, 1 ml samples were periodically withdrawn from the reactor through a sampl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com