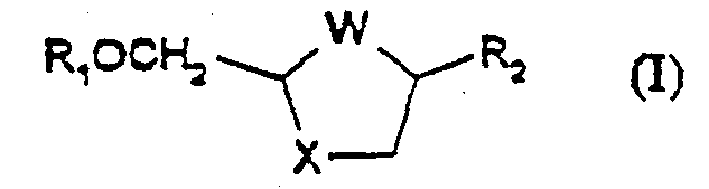
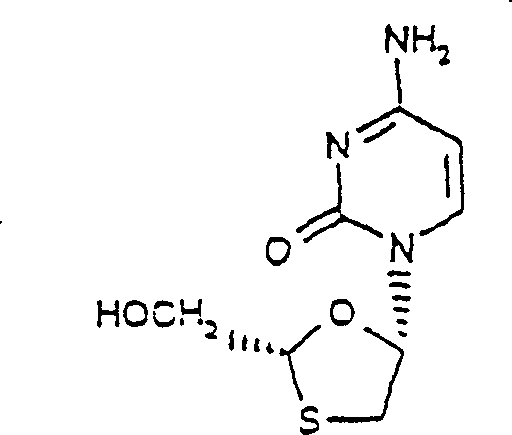
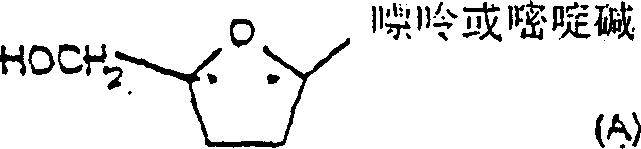Process for the diastereoselective synthesis of nucleoside analogues
A selective, enantiomeric technique, applied in organic chemistry and other directions, which can solve the problems of expensive, highly reactive, unstable compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] 4-amino-1-(2R-hydroxymethyl-[1,3]oxathiolane-5S-yl)- 1H-pyrimidin-2-one
[0109] (a) (2R,5R)-5-Hydroxy-[1,3]oxathiolane-2-carboxylic acid, 2S -Isopropyl-5R-methyl-1R-cyclohexyl ester
[0110] A mixture of l-menthylglyoxylate hydrate (25g) and acetic acid (2.5ml) in toluene (125ml) was stirred and heated at reflux. Water was removed by azeotropic distillation over a Dean-Stark trap. The resulting l-menthyl glyoxylate solution was concentrated by distillation under reduced pressure, and about 70 ml of distillate was collected, followed by cooling to 20-25°C. The volume was adjusted to 75ml by adding about 15ml of toluene, dithianediol (8.25g) was added and the mixture was heated at reflux for about 1 hour. The mixture was cooled to about 80°C and cleared. The filtrate was cooled to 0-5°C, a solution of triethylamine (1.5ml) in hexane (150ml) was added at 0-5°C in about 1.25 hours, and the resulting suspension was stirred at 0-5°C for 6 hours , and then, the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com