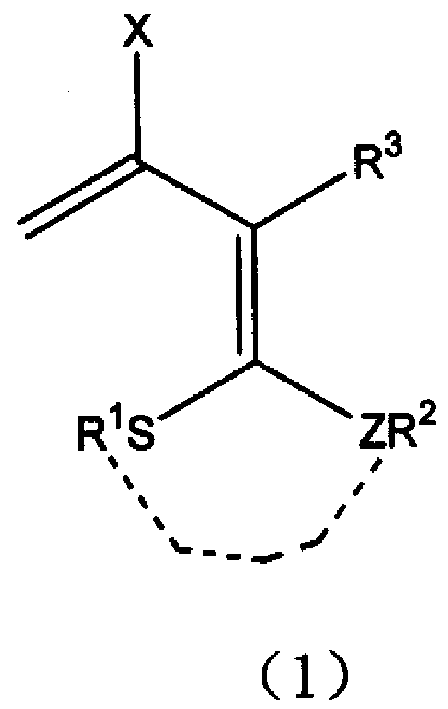
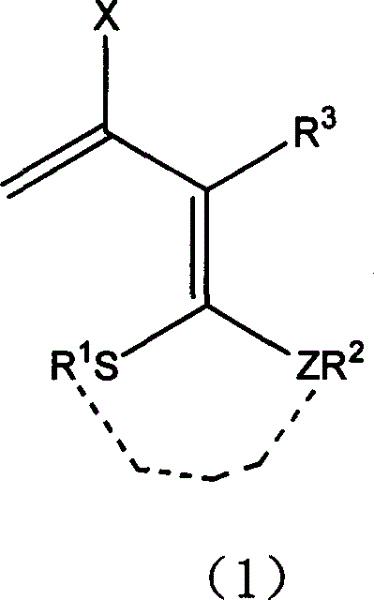
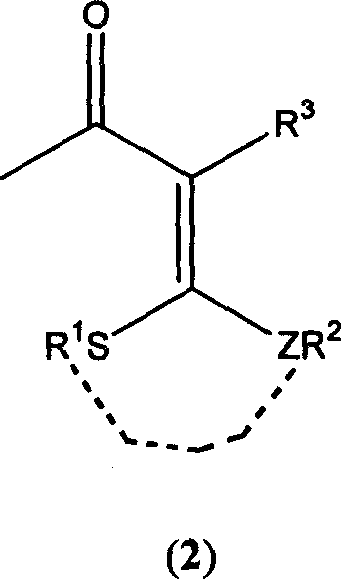
3-halo-1,1-bialkyl sulphide-1,3-butadiene derivatives and synthesis thereof
A technology of alkyl and alkoxy, which is applied in the field of carbonyl protection reagents in organic synthesis, which can solve the problems of high price, inconvenient transportation, and low-level mercaptans are easy to volatilize, and achieve the effect of low price, no peculiar smell, and convenient transportation and storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 30 ml of a mixed solvent of dimethylacetamide and ethyl acetate to a 100 ml three-necked flask equipped with a condenser tube, a constant pressure funnel, and a thermometer, and control the temperature at -5°C. Add 2 mmol of chlorine dropwise to the three-necked flask sulfoxide, the solution is slightly red at this time. After stirring for 30 minutes, add 2-[1,3]dithia-2-ylidene-3-carbonyl-butyric acid methyl ester (3) (232 mg, 1.0 mmol) in chloroform at one time, stir for 10 hours, and monitor the substrate by TLC disappear. The reaction solution was poured into ice water and stirred until a large amount of precipitates formed. After filtration, 208.46 mg of methyl 3-chloro-2-[1,3]dithia-2-ylidene-3-butenoate (4) was obtained, with a yield of 87%. (reaction see formula below).
[0021]
[0022] Structure Characterization:
[0023] Yield: 87% m.p.66-68°C 1 HNMR: 2.19 (2H, m, -CH 2 -), 2.99 (4H,t,-SCH 2 -), 3.78 (3H, s, -OCH 3 ), 5.33 (1H, d, J = 1.2 Hz,...
Embodiment 2
[0025] Add p-nitrobenzaldehyde (5) (151mg, 1.0mmol) and 3-chloro-2-[1,3]dithi-2-ylidene-3-butenoic acid methyl ester (4) (2mmol) In a round-bottomed flask equipped with a condenser, a mixture of absolute ethanol and tetrahydrofuran was used as a solvent. The reaction temperature was controlled at 65° C., stirred for 36 hours, and monitored by TLC until the substrate disappeared. The product 2-(4-nitrophenyl-[1,3]dithiane (6) 229mg was separated by column chromatography, and the yield was 95%. (See the following formula for the reaction).
[0026]
Embodiment 3
[0028] Add 1 mmol of 3-(bismethylthio)methylene-2,4-pentanedione (7) to a 100-ml three-necked flask equipped with a condenser tube, a constant pressure funnel, and a thermometer, and mix it with benzene, ether, and DMF. Dissolve it in 40 ml of solvent, control the temperature at 0°C, and add 4 mmol of phosphorus trichloride dropwise to the three-necked flask, and the solution is slightly red at this time. After stirring at room temperature for 5 hours, the color of the system deepened. TLC monitoring until the disappearance of the substrate. The reaction solution was poured into ice water and stirred until a large amount of precipitates formed. The product 3-(bismethylthio)methylene-2,4-dichloro-1,4-pentadiene (8) was filtered to obtain 236mg, and the yield was 98%. (reaction see formula below).
[0029]
[0030] Structure Characterization:
[0031] Yield: 98% m.p: 38-40°C 1 HNMR: 2.39 (6H, s, -SCH 3 ), 5.43 (2H, s, =-H), 5.64 (2H, s, =-H), IR: 3103, 2922, 1618, 150...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com