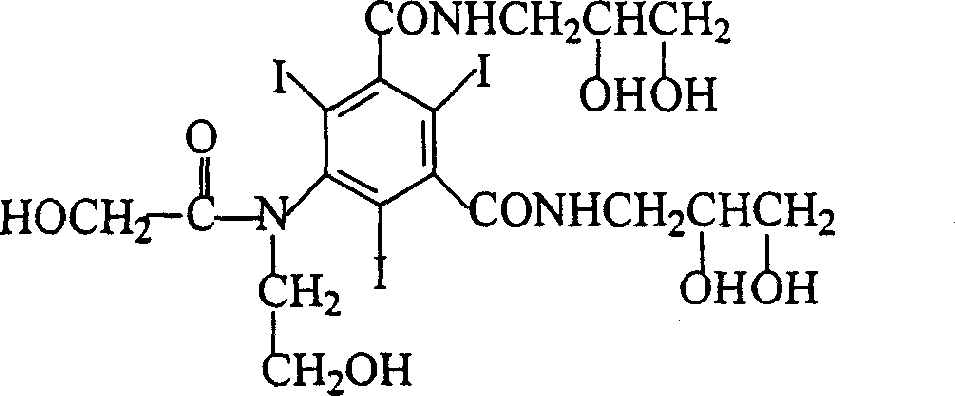
Method for purifying ioversol
A purification method and technology of ioversol, applied in the separation/purification of carboxylic acid amides, organic chemistry and other directions, can solve the problems such as the inability to purify ioversol completely
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a 3L three-necked flask equipped with an electric stirrer and reflux condenser, add the crude ioverol (45g) to n-butanol (2250mL), gradually increase the temperature to reflux with stirring, reflux for 5 hours, and then filter the white while hot The suspension was washed with hot n-butanol (3×15 mL) on the filter and the crystals were dried in vacuum to obtain 27.5 g of ioverol. HPLC analysis (water / acetonitrile, C 8 column). The results are shown in Table 1.
[0022] Dissolve ioverol (27.5g) obtained from the first recrystallization above in water (150mL), evaporate to dryness under reduced pressure, add n-butanol (1375mL), carry out the second recrystallization, the operation steps are the same as the first recrystallization Crystallized to obtain 19.3 g of ioverol, which was analyzed by HPLC (water / acetonitrile, C 8 column). The results are shown in Table 1.
[0023] Peak
Embodiment 2
[0025] In a 1L three-necked flask equipped with an electric stirrer and a reflux condenser, add the crude ioverol (45g) to the mixture of 2-methoxyethanol (135mL) and isopropanol (540mL), and gradually raise the temperature to Keep the temperature constant at 100°C for 5 hours, then filter the white suspension while hot, wash the crystals with hot isopropanol (3×15 mL) on the filter, and dry in vacuo to obtain 29.2 g of ioverol. HPLC analysis (water / acetonitrile, C 8 column). The results are shown in Table 2.
[0026] Dissolve ioverol (29.2g) obtained from the first recrystallization in water (150mL), evaporate to dryness under reduced pressure, add 2-methoxyethanol (88mL) and isopropanol (350mL) for the second time Recrystallization, the procedure is the same as the first recrystallization, 20.8g of ioverol was obtained, which was analyzed by HPLC (water / acetonitrile, C 8 column). The results are shown in Table 2.
[0027] Peak
Embodiment 3
[0029] In a 1L three-necked flask equipped with an electric stirrer and a reflux condenser, add the crude ioverol (45g) to the mixture of 2-methoxyethanol (90mL) and n-butanol (600mL), and gradually increase the temperature to 100 with stirring. Keep the temperature constant for 5 hours, then filter the white suspension while hot, wash the crystals with hot n-butanol (3×15 mL) on the filter, and dry in vacuum to obtain 31.5 g of ioverol. HPLC analysis (water / acetonitrile, C 8 column). The results are shown in Table 3.
[0030] Dissolve ioverol (31.5g) obtained from the first recrystallization in water (150mL), evaporate to dryness under reduced pressure, add 2-methoxyethanol (63mL) and n-butanol (420mL) for the second recrystallization Crystallization, the operation steps are the same as the first recrystallization, and 25g ioverol is obtained, and analyzed by HPLC (water / acetonitrile, 8 column). The results are shown in Table 3.
[0031] Peak
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com