Transition metal sulfied nano-pipe and preparation process and its application
A technology of transition metals and nanotubes, applied in chemical instruments and methods, molybdenum sulfide, niobium compounds, etc., to achieve high-rate discharge, excellent high-temperature performance, small volume expansion, and the effect of inhibiting the formation of lithium dendrites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: high-purity MS 2 Low-temperature catalytic preparation of nanotubes.
[0021] High-purity TiS 2 , ZrS 2 , NbS 2 Low-temperature catalytic preparation of nanotubes. In an Ar gas glove box, 5 g of TiS with a purity of 99% 3 , ZrS 3 or NbS 3 The crystal powder and 25g stainless steel balls (Φ6mm) were put into a stainless steel tank, and sealed with a cover. The sealed stainless steel tank was placed on a planetary ball mill (Nanjing University Instrument Factory, QM-1SPR-CL) for high-energy ball milling (rotation speed: 600 rpm) for 1 hour. Put the ball-milled powder on the Al 2 o 3 On the thin slice, put it into the high-temperature reaction furnace. In the experiment, H was introduced at a volume ratio of 18:1:1 2 / CH 4 / C 4 h 4 S (total flow 100mL / min), heat reaction at 360°C for 1h. The reaction tail gas is introduced into 1M ZnSO 4 solution to remove the generated gas H 2 S. The thermal decomposition reactions involved in its growth are:...
Embodiment 2
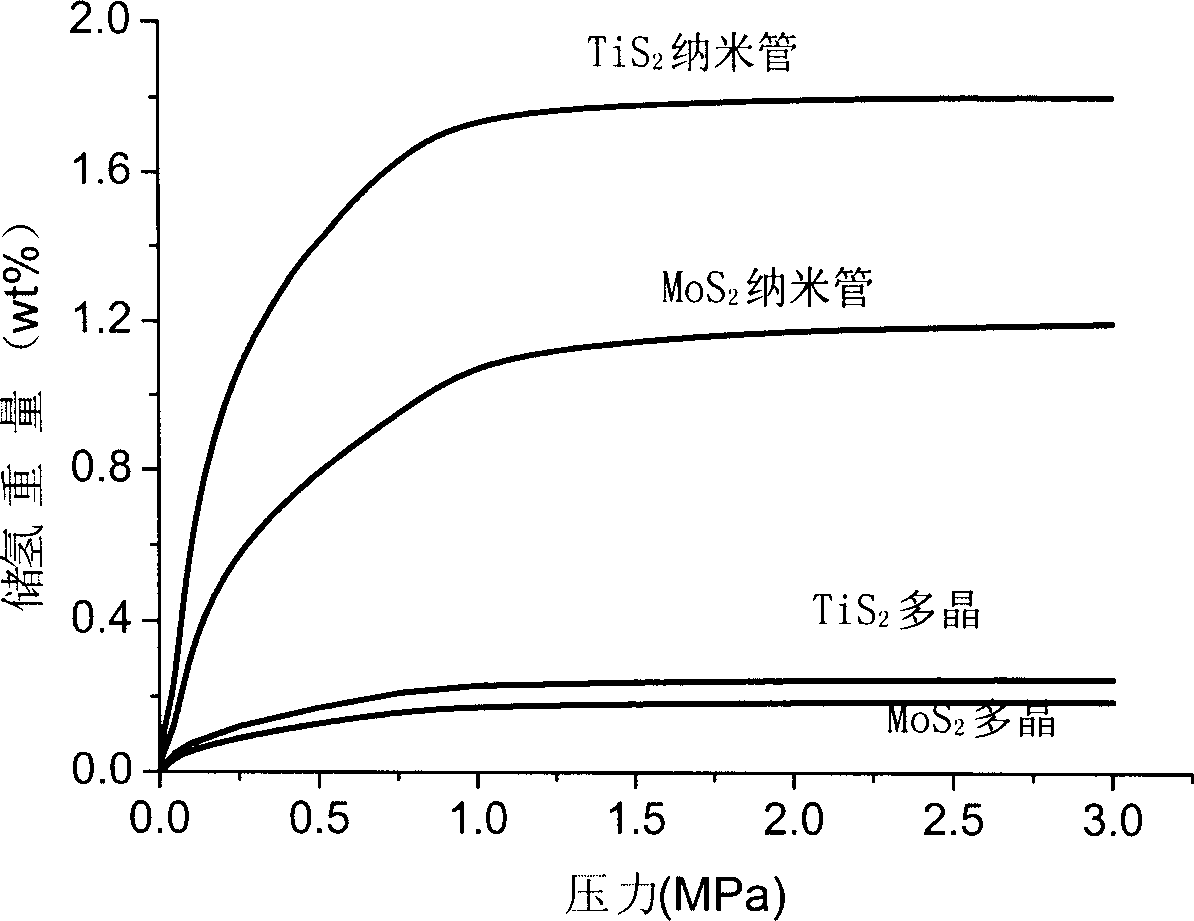
[0027] TiS prepared according to embodiment 1 2 and MoS 2 Nanotubes outperform ordinary TiS in terms of gas / solid hydrogen storage capacity 2 and MoS 2 Polycrystalline materials have been greatly improved. TiS was measured using a pressure / composition / temperature (PCT) device 2 and MoS 2 Hydrogen storage comparison between nanotubes and polycrystalline materials image 3 shown. After reaching the hydrogen absorption platform, TiS 2 The hydrogen uptake of nanotubes is 1.8wt%, MoS 2 The hydrogen absorption capacity of the nanotubes is 1.2wt%, while the TiS 2 and MoS 2 The hydrogen uptake of polycrystalline materials is only 0.2wt% and 0.25wt%, respectively. Therefore, using TiS 2 and MoS 2 The capacity of nanotubes for gas / solid hydrogen storage is significantly higher than that of their polycrystalline counterparts.
Embodiment 3
[0028] Example 3: TiS prepared in Example 1 by using an electrochemical three-electrode measurement system 2 and MoS 2 The nanotubes were analyzed for electrochemical hydrogen storage. The electrochemical hydrogen storage capacity data are shown in Table 1. The results showed that: TiS 2 and MoS 2 The discharge capacity of nanotubes is significantly higher than their polycrystalline counterparts, and the high-rate discharge performance of nanotubes is also good. After 100 0.5C charge / discharge cycles, the TiS 2 and MoS 2 The electrode capacity of nanotubes decays only about 2% ( Figure 4 ). Therefore, such nanotubes can be applied to the development of new nickel / hydrogen batteries.
[0029] Electrode material
[0030] Note: The high rate discharge performance refers to the ratio of the capacity of the electrode discharged at 200mA / g and 50mA / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com