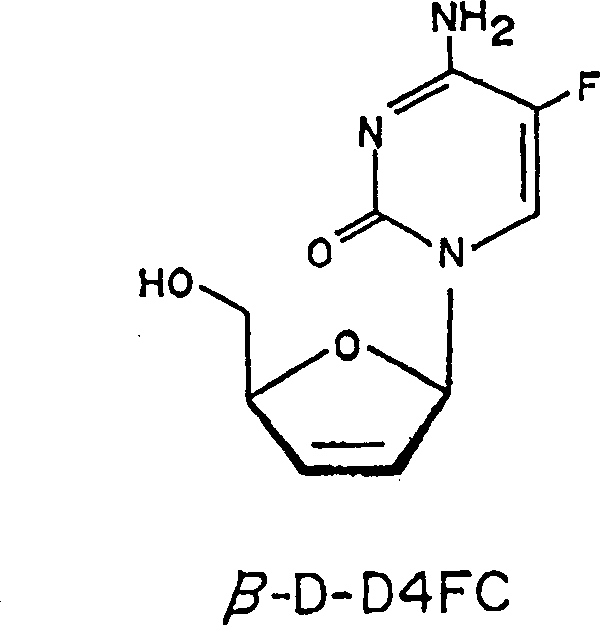
HIV-1 mutation selecting from beta-2',3'-didehydro-2',3-dideoxy-5-fluorocytidine
A HIV-1, dideoxythymidine technology, applied in the preparation of mutants, medical preparations containing active ingredients, antibacterial drugs, etc., can solve the problem of unpredictable induction of which mutations are permanent or temporary, and exacerbate unpredictability. , the lack of kinetic parameters of drug resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0015](i) a method of treating HIV infections in the human body, including β-D-D4FC or pharmaceutically acceptable precursor or salt thereof or salt thereof to the person to give an effective amount to the human body, with one in HIV- 1 In the case where the mutation is induced or alternately induced by a drug in which the mutation is induced by the 70 (k mutant to n), 90 or 172-bit codon in the reverse transcription enzyme region, the drug is not a cis-2-hydroxymethyl group. -5- (5-fluoroamidine-1-yl) -1,3-oxide ring, 顺 -2-hydroxymethyl-5- (cytosine-1-yl) -1,3-oxygen Ring, 9- [4- (hydroxymethyl) -2-cyclopentene-1-yl]-guanine (carbovir), 9- [2- (hydroxyethoxy) methyl] - guanine Lown, Interferon, 3'-deoxidation-3'-azide-thymidine (AZT), 2 ', 3'-diboxide (DDI), 2', 3'-diodeobide ( DDC), (-) - 2'-fluoro-5-methyl-β-L-Arabicanurine (L-FMAU) or 2 ', 3'-dihydrophyde-2', 3'-di deoxyridine (D4T).
[0016] (ii) a method of treating HIV infections in the human body, including β-D-D4FC or pha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com