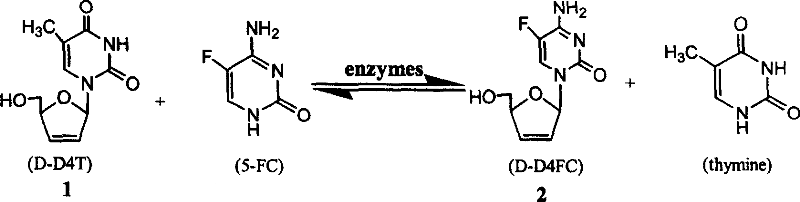
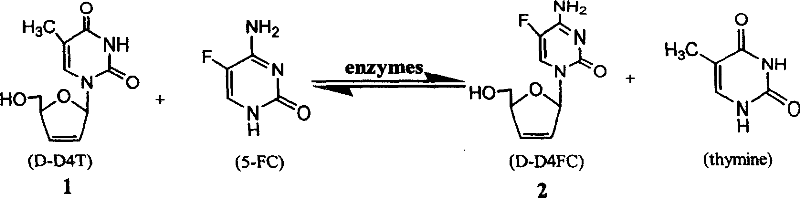
Synthesis method of 5-fluorocytidine
A technology of flucytosine nucleoside and synthesis method, which is applied in fermentation and other directions, can solve the problems of high synthesis cost, long synthesis route, and low photoactivity, and achieve shortened synthesis steps, less side reactions, and good reaction selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Take unsaturated thymidine (D-D4T) 1112mg (50mmol), 5-fluorocytosine (5-FC) 124mg (100mmol), KH 2 PO 4 30mL of buffer solution (pH6.35), 4ml of self-made deoxyribose transferase solution in a 100ml Erlenmeyer flask, shake in a constant temperature water bath at 150r / min for 20-24h. The reaction was boiled in boiling water for 5 min to stop the reaction. The reaction solution was lyophilized, and the residue was extracted with 10 ml of methanol / dichloromethane, and the extract was developed by TLC to obtain 214.96 mg of the product, Y: 16.8%.
Embodiment 2
[0020] Take unsaturated thymidine (D-D4T) 1112mg (50mmol), 5-fluorocytosine (5-FC) 124mg (100mmol), KH 2 PO 4 30mL of buffer solution (pH6.35), 4ml of self-made deoxyribose transferase solution in a 100ml Erlenmeyer flask, shake in a constant temperature water bath at 150r / min for 20-24h. The reaction was boiled in boiling water for 5 min to stop the reaction. The reaction solution was lyophilized, the residue was extracted with 30 ml of methanol, and concentrated under reduced pressure to obtain a solid mixture. The solid mixture was subjected to silica gel chromatography (20% CH 3 OH / CH 2 Cl 2 , v / v) purification to obtain 227.5 mg of the product, Y: 24.6%.
Embodiment 3
[0022] Take unsaturated thymidine (D-D4T) 1112mg (50mmol), 5-fluorocytosine (5-FC) 124mg (100mmol), citric acid buffer 30mL (pH6.35), 4ml homemade deoxyribose transferase solution in a 100ml Erlenmeyer flask, shaken at 150r / min in a constant temperature water bath for 20-24h. The reaction was boiled in boiling water for 5 min to stop the reaction. The reaction solution was lyophilized, the residue was extracted with 30 ml of methanol, and concentrated under reduced pressure to obtain a solid mixture. The solid mixture was subjected to silica gel chromatography (20% CH 3 OH / CH 2 Cl 2 , v / v) purification to obtain 227.0 mg of the product, Y: 24.1%.
[0023] Qualitative analysis during the reaction was performed by TLC. Activated silica gel G plate was used as carrier, dichloromethane-methanol-water (8:2:0.25v / v) was used as developer, and ultraviolet color was developed. R of D-D4T, D-D4FC, 5-FC and thymine f The reference values are 0.82, 0.61, 0.29 and 0.05 respective...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com