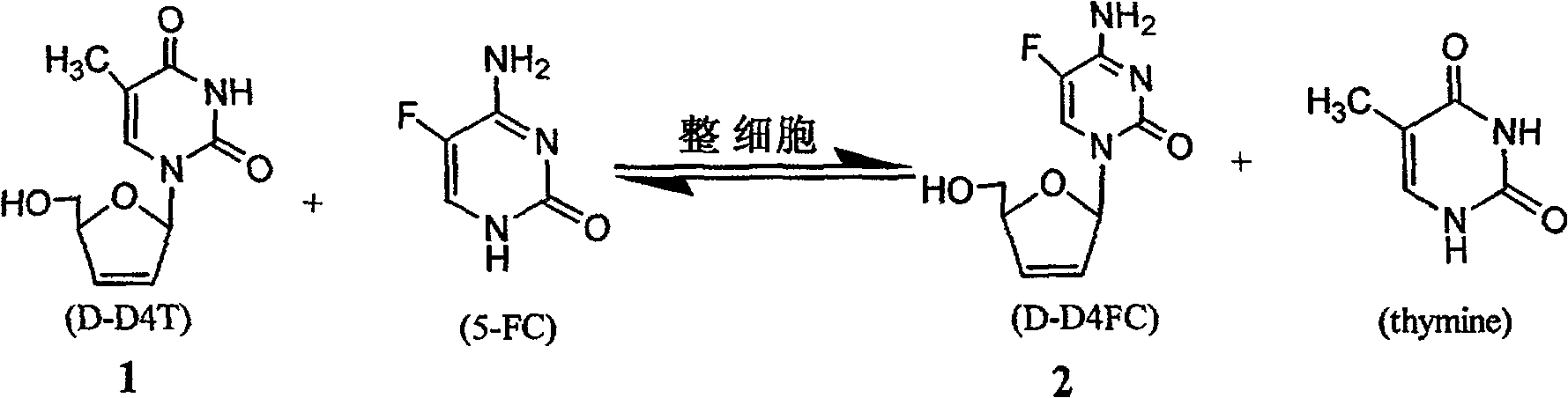
5-fluorocytidine complete cell enzymatic synthesis method
A flucytosine and enzymatic synthesis technology, which is applied in the field of 5-fluorocytosine synthesis, can solve the problems of long time consumption, low overall yield, unfavorable large-scale continuous production and the like, and achieves improved yield, The effect of shortened time and convenient recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 1mL 50mmol / / L NaH with pH=8 2 PO 4 Add D-D4T 5.6mg (25mmol / L), 5-FC 6.4mg (50mmol / L), and 1.2g Lactobacillus into a 5mL Erlenmeyer flask to the buffer, and seal it with multiple layers of gauze. The reaction was shaken at 120r / min in an air shaker at 40°C for 12.5h. After the reaction was completed, the reaction system was frozen and stored at -20°C to terminate. After the sample was thawed, it was centrifuged at 10,000 r / min for 15 minutes to remove the whole cells, and the obtained supernatant was filtered and used for HPLC detection.
Embodiment 2
[0019] 1mL 50mmol / / L NaH with pH=6.85 2 PO 4 Add 5.6mg (25mmol / L) of D-D4T, 6.4mg (50mmol / L) of 5-FC, and 2g of Lactobacillus to the buffer solution in a 5mL Erlenmeyer flask, and seal it with multiple layers of gauze. The reaction was shaken at 120r / min in an air shaker at 40°C for 12.5h. After the reaction was completed, the reaction system was frozen and stored at -20°C to terminate. After the sample was thawed, it was centrifuged at 10,000 r / min for 15 minutes to remove the whole cells, and the obtained supernatant was filtered and used for HPLC detection.
Embodiment 3
[0021] 1mL 50mmol / / L NaH with pH=6.85 2 PO 4 Add 5.6mg (25mmol / L) of D-D4T, 6.4mg (50mmol / L) of 5-FC, and 1.2g of Lactobacillus to the buffer solution in a 5mL Erlenmeyer flask, and seal it with multiple layers of gauze. The reaction was shaken at 120r / min in an air shaker at 40°C for 100h. After the reaction was completed, the reaction system was frozen and stored at -20°C to terminate. After the sample was thawed, it was centrifuged at 10,000 r / min for 15 minutes to remove the whole cells, and the obtained supernatant was filtered and used for HPLC detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com