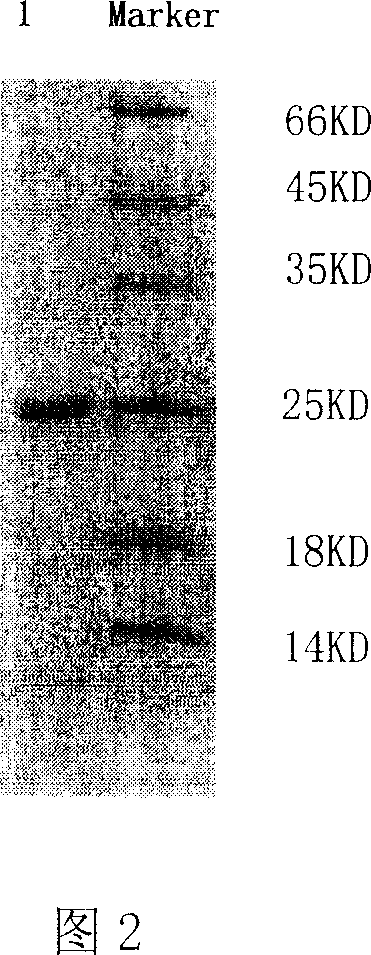Snake venom thrombin-like enzyme and its encoding gene and application
A thrombin-like, gene-encoding technology, applied in applications, genetic engineering, plant genetic improvement, etc., can solve problems such as limited yield and achieve broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
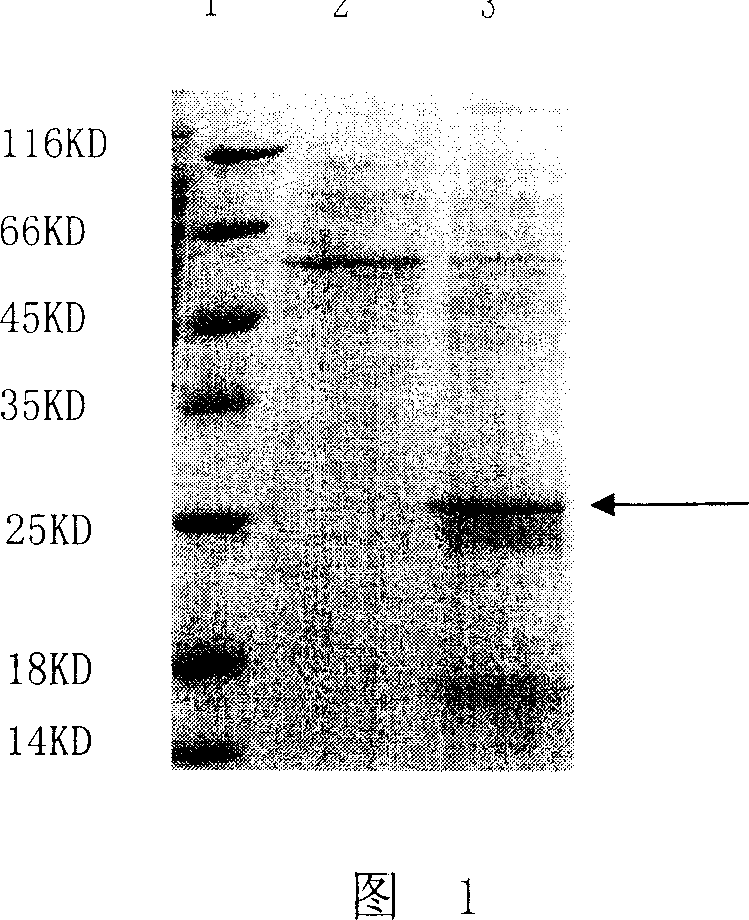
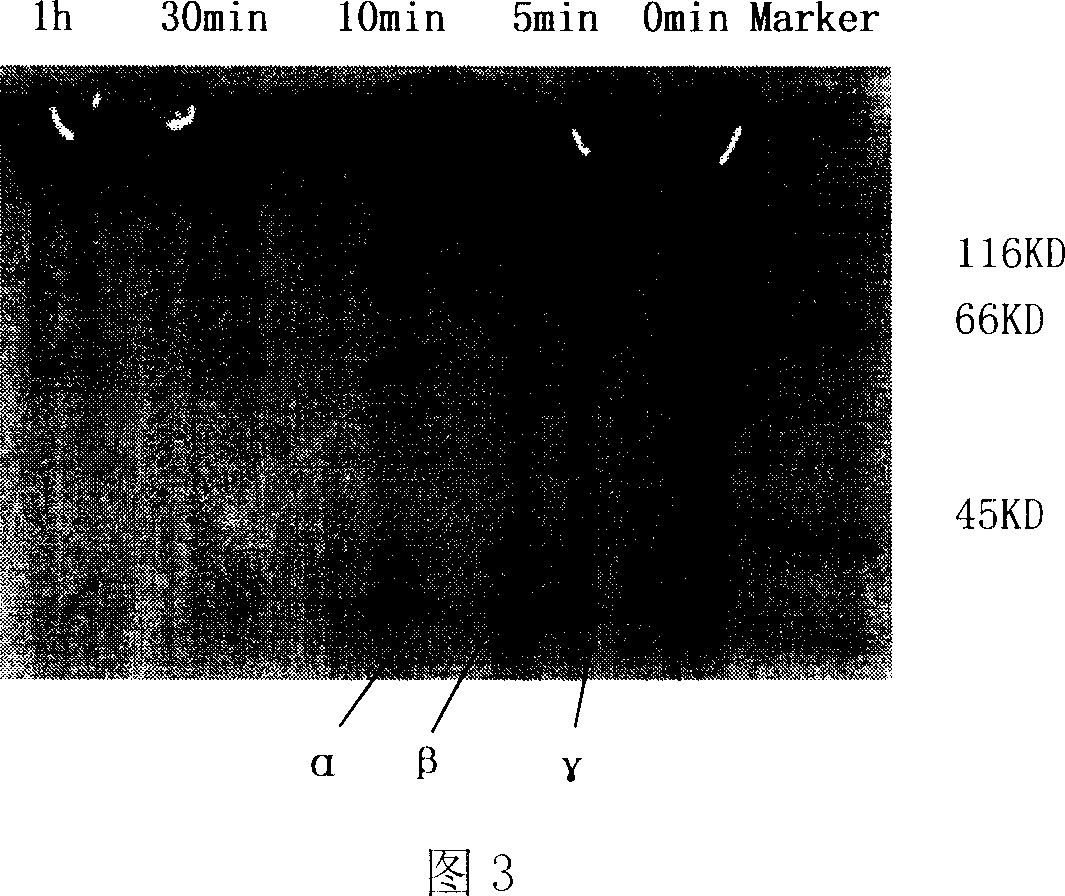
Image
Examples
Embodiment 1
[0051] Example 1. Obtaining of TLE and expression of snake venom thrombin-like mature protein
[0052] 1. Obtaining TLE
[0053] Include the following steps:
[0054] 1) The venom gland tissue was isolated from the flat-chin sea snake (Lapemis curtus) and the blue-gray sea snake (Hydrophiscaerulescens) captured in the Beibu Gulf of China, and the total RNA of the venom of the two sea snakes was extracted;
[0055] 2) extracting mRNA from the total RNA of the two kinds of sea snake venoms, and using the mRNA as a template to reverse transcribe and synthesize their respective cDNA sequences;
[0056] 3) Ligate the two synthetic cDNAs with the T7 phage vector (Novagen) respectively, and obtain the sea snake phage display polypeptide library through in vitro packaging;
[0057] 4) ELISA method was used to screen the phage display peptide library constructed in step 3) with fibrinogen (fibrinogen, sigma company) as the target molecule, and phage clones with specific binding abili...
Embodiment 2
[0066] Embodiment 2, detection of enzyme activity of snake venom thrombin-like mature protein
[0067] 1. Determination of amidolysis activity
[0068] At 37°C, add a chromogenic substrate (N-α-p-tosyl-Gly-Pro-Arg-p-nitronilide, Sigma Company) to a concentration of 2mM in 50mM phosphate buffer (pH8.0) and Example 1 The obtained snake venom thrombin-like mature protein to a concentration of 1mg / mL, after reacting for 10min, by detecting OD 405 The changes of the snake venom-like thrombin mature protein to measure the amidolysis activity, the experimental results show that the snake venom-like thrombin mature protein can degrade the chromogenic substrate, with amidolysis activity (1062.5 enzyme activity U / min / mg enzyme), It shows that the thrombin-like mature protein of the snake venom is similar to the thrombin-like enzymes of other land snakes, and both have amidolysis activity, which can degrade the amide bond in the protein polypeptide.
[0069] 2. Determination of arginin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com