Modified factor VIII
A technology for coagulation factor and human coagulation factor, which is applied in the directions of coagulation/fibrinolysis factor, factor VII, blood diseases, etc., and can solve the problems of difficult separation and purification, immunogenicity, insufficient supply of factor VIII, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 4
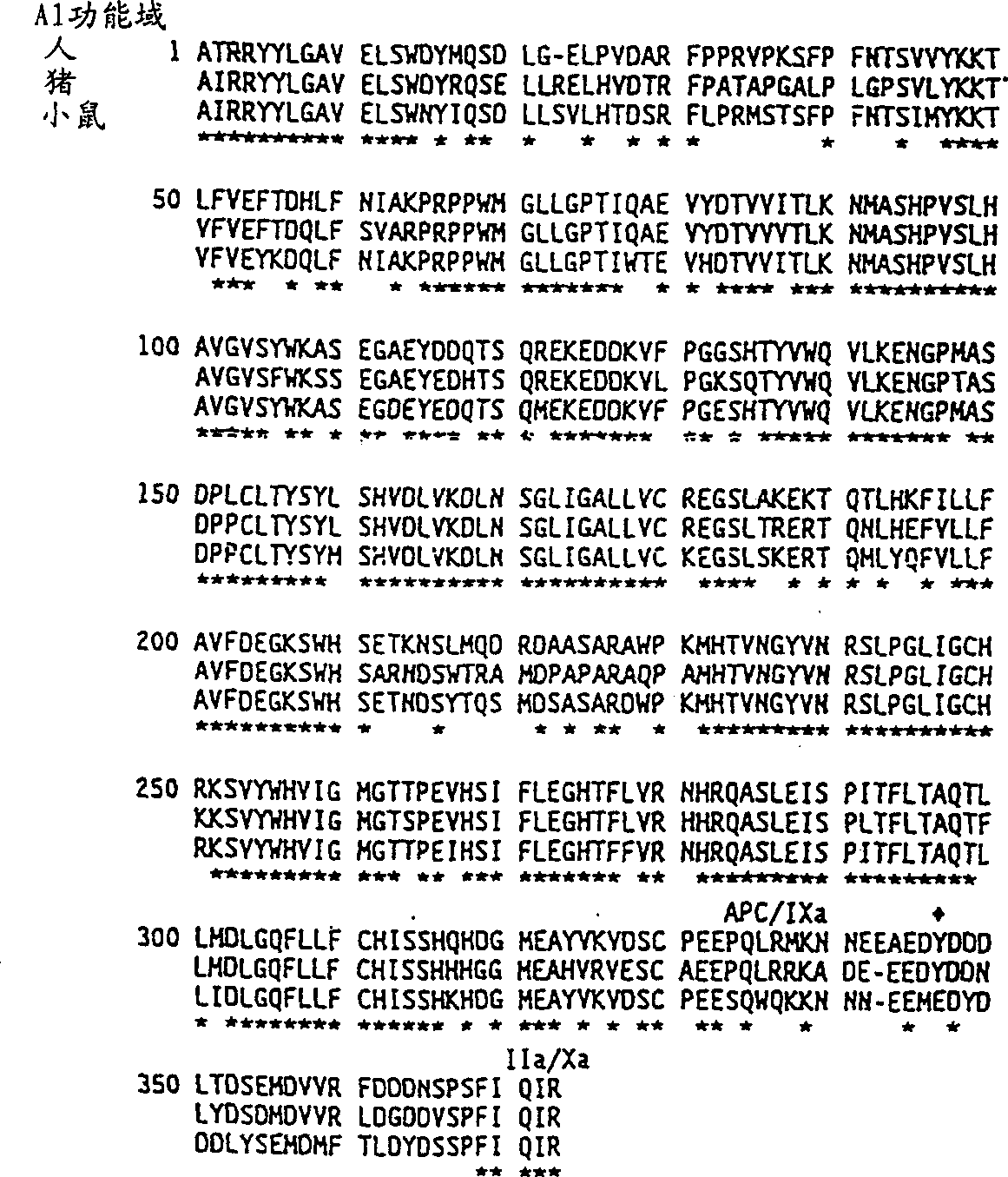
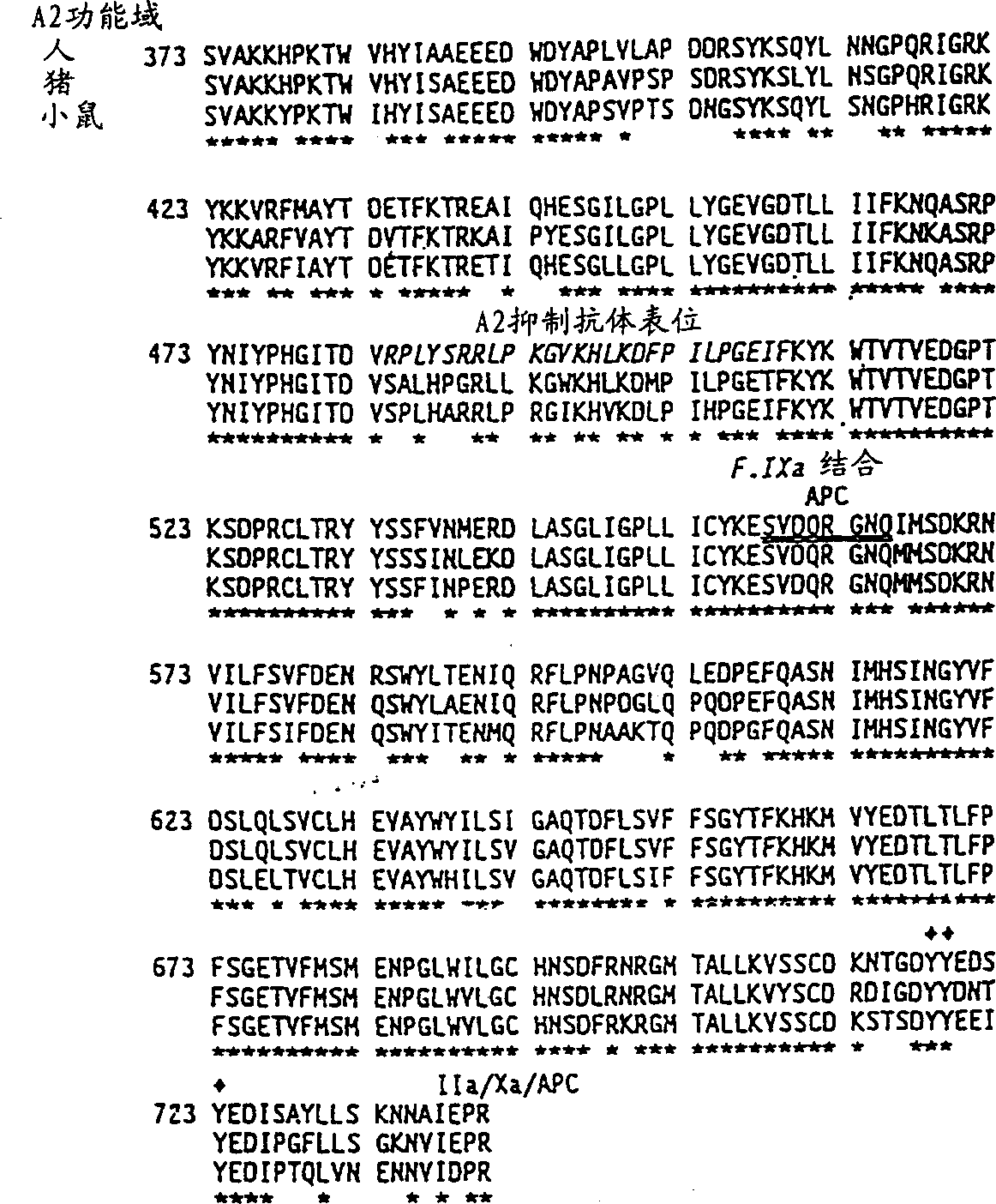
[0050] It is shown in Example 4 that a hybrid human / porcine factor VIII, which contains a porcine heavy chain and a human light chain, and corresponds to the Type 1 cross listed above, has better coagulation assays compared to human factor VIII. Strong specific coagulation activity. Hybrid human / animal or equivalent coagulation factor VIII with coagulation activity, whether higher, equal, or lower than human coagulation factor VIII, are useful in the treatment of patients with inhibitory antibodies because these inhibitory antibodies and hybrid human / Animal or equivalent factor VIII response is weaker than human or porcine factor VIII.
[0051] Hybrid factor VIII molecules were prepared by recombination from isolated human and animal factor VIII subunits:
[0052] The present invention provides subunit-substituted hybrid human / animal coagulation factor VIII molecules or fragments thereof, nucleic acid sequences encoding these hybrids, methods of making and isolating them, a...
Embodiment 1
[0160] Example 1: Test of porcine blood coagulation factor VIII and hybrid human / pig coagulation factor VIII
[0161] According to the specific activity of this molecule, porcine coagulation factor VIII has stronger coagulation activity than human coagulation factor VIII. These results are shown in Table III of Example 4. The results were based on the use of appropriate standard curves to allow unbiased comparisons between human and porcine factor VIII. Coagulation tests are based on the ability of coagulation factor VIII to shorten the plasma clotting time in patients with hemophilia A. Two tests were used: primary and secondary tests.
[0162] In a primary test, 0.1 mL of hemophilia A plasma (George King Biomedical, Inc.) and 0.1 mL of activated partial thromboplastin (APTT) (Organon Teknika) were mixed with 0.01 mL of sample or containing diluted Citrated normal human plasma standards were incubated together in a 37°C water bath for 5 minutes. Then add 0.1 ml of 20 mM C...
Embodiment 2
[0165] Example 2: Characterization of functional differences between human and porcine coagulation factor VIII
[0166] The separation of porcine and human plasma-derived coagulation factor VIII and human recombinant coagulation factor VIII is described in literature such as Fulcher, C.A. et al. (1982) Proc.Natl.Acad.Sci.USA 79:1648-1652; Toole et al. (1984) Nature 312: 342-347 (Genetics Institute); Gitschier et al. (1984) Nature 312:326-330 (Genentech); Wood et al. (1984) Nature 312:330-337 (Genentech); Vehar et al., Nature 312:337-342 (Genentech) ; Fass et al. (1982) Blood 59:594; Toole et al. (1986) Proc. Natl. Acad. Sci. USA 83:5939-5942. This can be done in several ways. All of these preparations were similar in subunit composition, despite functional differences in stability between human and porcine factor VIII.
[0167] To compare human recombinant and porcine factor VIII, highly purified human recombinant factor VIII (Cutter Laboratories, Berkeley, CA) and porcine f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com