Synthesis of seleno-methionine
A technology of selenomethionine and its synthesis method, which is applied in the field of selenium-containing compounds, can solve the problems of low yield, large equipment investment, and difficulty in industrial production, and achieve the effects of simple steps, mild reaction conditions, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
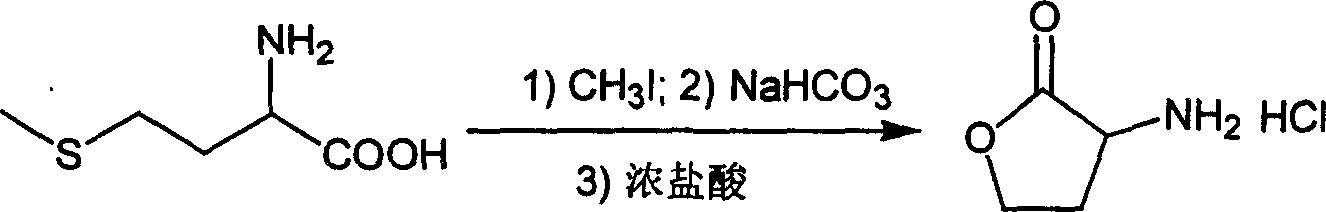
[0015] The preparation of step (1), α-aminobutyrolactone hydrochloride
[0016] 75g (0.50mol) of methionine was added to methanol aqueous solution (methanol / water=200:1400), and 75mL (1.21mol) of methyl iodide was added under stirring. Stir the reaction for 48 hours, distill and concentrate the reaction solution to about 1 / 3 of the original volume, add 500mL water, 42g (0.5mol) NaHCO 3 Hydrolyze the aqueous solution, heat to reflux for 15 hours, cool, distill off the solvent, add 1000mL of concentrated hydrochloric acid to acidify, and add 25mL of 30% H 2 o 2 , stirred and reacted for 1 hour, extracted with ether to remove iodine, continued to reflux the water phase for 1 hour, cooled, distilled off the solvent, extracted the residual solid with ethanol several times, added 1000 mL of concentrated hydrochloric acid after concentration of the extract and heated to reflux for 1 hour, distilled off Solvent, the crude product was recrystallized with ethanol / water to obtain 50.6 ...
Embodiment 2
[0020] Step (1), with embodiment 1.
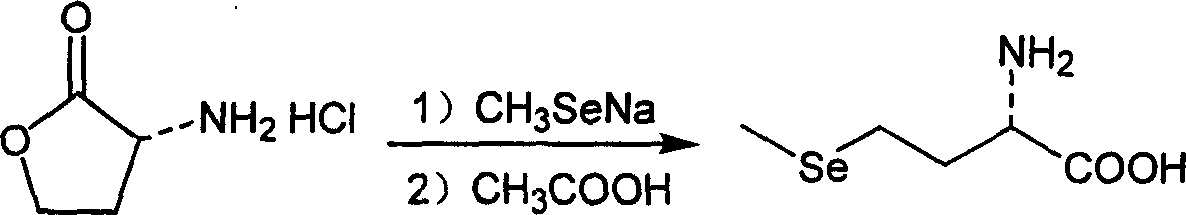
[0021] Step (2), under nitrogen protection and stirring, the α-aminobutyrolactone hydrochloride 7.74g (0.056mol) prepared above was added in the absolute ethanol solution of sodium methylselenide 12.41g (0.084mol), Heat to reflux for 8 hours, cool, add acetic acid to neutralize until the pH value is 5.75, and filter to obtain a white product, selenomethionine, 5.85 g, with a yield of 53%.
Embodiment 3
[0023] Step (1), with embodiment 1.
[0024] Step (2), under nitrogen protection and stirring, the α-aminobutyrolactone hydrochloride 7.74g (0.056mol) prepared above was added in the absolute ethanol solution of sodium methylselenide 20.68g (0.141mol), Heating to reflux for 8 hours, cooling, adding acetic acid to neutralize to a pH value of 5.75, and filtering to obtain a white product, selenomethionine, 8.29 g, with a yield of 74%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com